The key difference between thermochemical equation and chemical equation is that a thermochemical equation shows the enthalpy change of the reaction, whereas a chemical equation does not usually show the enthalpy change.
A thermochemical equation is a balanced stoichiometric chemical reaction that includes the enthalpy change, while a chemical equation is an equation showing the starting compound, reactants, and final products separated by an arrow.
CONTENTS
1. Overview and Key Difference
2. What is a Thermochemical Equation
3. What is a Chemical Equation
4. Thermochemical Equation vs Chemical Equation in Tabular Form
5. Summary – Thermochemical Equation vs Chemical Equation
What is a Thermochemical Equation?
A thermochemical equation can be described as a balanced stoichiometric chemical reaction that indicates the enthalpy change. The enthalpy change is denoted by ΔH. In its variable form, this type of reaction appears as A + B → C; ΔH = (±) # where A and B are reactants, C is the final product, and (±) # is the positive or negative numerical values for enthalpy change.
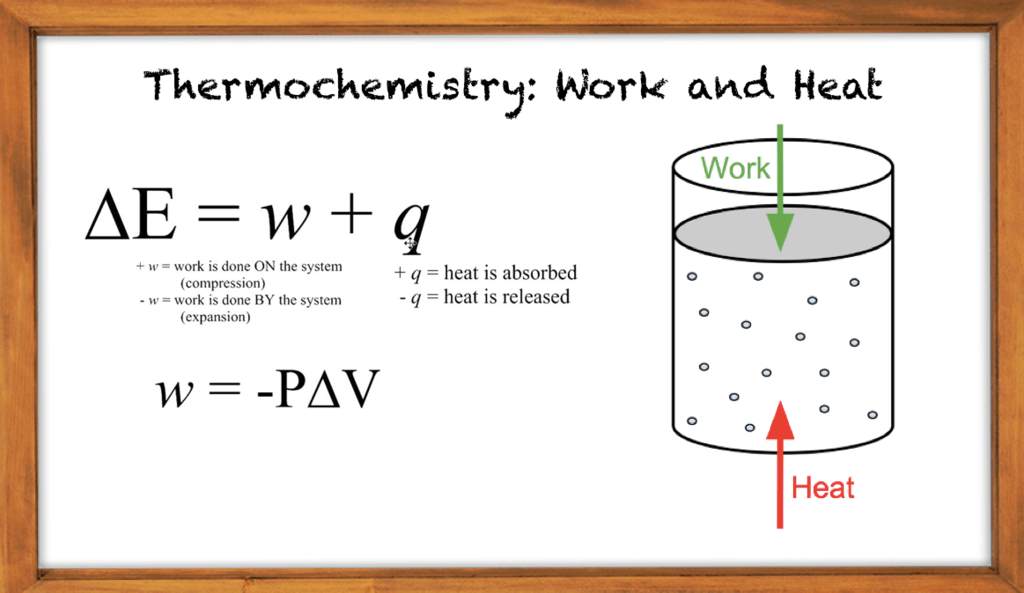
Enthalpy of a system is a thermodynamic quantity equivalent to the total heat content of a system. It is equal to the internal energy of the system plus the product of pressure and volume. Therefore, it is a thermodynamic property of a system.
We can change a thermochemical equation by multiplying it by any numerical coefficient. In this method, we need to multiply all the agents, including the enthalpy change. For example, the above-given example can be multiplied by “2,” and it gives 2A + 2B → 2C, 2ΔH = 2[(±) #].
What is a Chemical Equation?
A chemical equation is an equation showing the starting compound, reactants, and final products separated by an arrow. In other words, a chemical equation is a representation of a chemical reaction. This means the chemical equation gives the reactants of the reaction, the end product, and the direction of the reaction as well. There are two types of equations: balanced equations and skeleton equations.
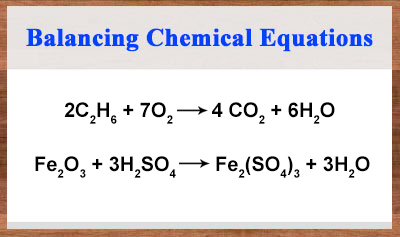
A balanced chemical equation gives the actual number of reactants that react with each other and the number of product molecules formed. It is a fully detailed equation that indicates the ratios between reactants and products. When calculating a parameter such as the amount of product we obtain from the reaction, we have to use the balanced chemical equation; otherwise, we won’t know how much reactants reacted to give how much of products.
A skeleton equation indicates the types of reactants involved in the chemical reaction and the end products. However, this does not give the exact ratio between the reactants and products. Therefore, the important details we can get from a skeleton equation are the reactants of the reaction, the products of the reaction, and the direction of the reaction.
What is the Difference Between Thermochemical Equation and Chemical Equation?
In chemistry, an equation shows the molecules involved in a particular chemical reaction and the direction of the reaction progression. The key difference between thermochemical equation and chemical equation is that a thermochemical equation always shows the enthalpy change of the reaction, whereas a chemical equation does not generally show the enthalpy change.
The below infographic presents the differences between thermochemical equation and chemical equation. in tabular form for side-by-side comparison
Summary – Thermochemical Equation vs Chemical Equation
A thermochemical equation is a balanced stoichiometric chemical reaction that includes the enthalpy change, whereas a chemical equation is an equation showing the starting compound, reactants, and final products separated by an arrow. The key difference between thermochemical equation and chemical equation is the enthalpy change of the reaction. Thermochemical equations always indicate the enthalpy change of reactions, but chemical equations do not typically show the enthalpy change.
Reference:
1. “3.1: Chemical Equations.” Chemistry LibreTexts, Libretexts, 8 Nov. 2021.
Image Courtesy:
1. “Tchdc” By 467 Divya.N – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Balancing Chemical Equation” By Zappys Technology Solutions (CC BY 2.0) via Flickr
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoytn56qnaSwqbHMopqapF2avrat06Kmp2WRo7Fur8eepKKbkaF6pr3Umquip55k