The key difference between sulfite and sulfur trioxide is that sulfite is an ionic compound having the sulfate (IV) anion whereas sulfur trioxide is a non-ionic compound.
Sulfite and sulfur trioxide are chemical compounds containing sulfur atoms. The term sulfites refer to ionic compounds containing sulfite anion bound to different cations. Sulfur trioxide is an inorganic compound having the chemical formula SO3.
CONTENTS
1. Overview and Key Difference
2. What is Sulfite
3. What is Sulfur Trioxide
4. Sulfite vs Sulfur Trioxide in Tabular Form
5. Summary – Sulfite vs Sulfur Trioxide
What is Sulfite?
The term sulfites refer to ionic compounds containing sulfite anion bound to different cations. The chemical formula for sulfite anion is SO32-. It is also named as sulfate (IV) ion where sulfur atom in the anion has +4 oxidation state. The sulfite anion is the conjugate base of bisulfite. Sulfite compounds usually occur in some foods and also inside the human body. Furthermore, sulfites are useful as food additives and can form lumps when they occur together with sulfur dioxide in food.
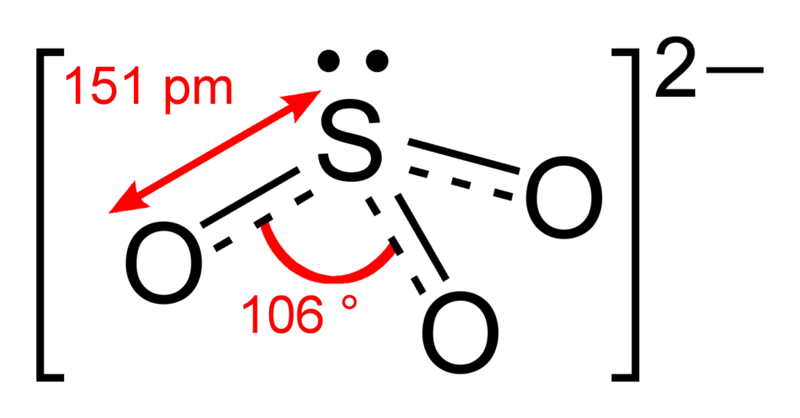
Figure 01: Structure of Sulfite Anion
There are three possible resonance structures for sulfite anion. In each resonance structure, we can observe that the sulfur atom is double-bonded to one of three oxygen atoms. Therefore, each resonance structure has a sulfur to oxygen double bond with a formal charge of zero, while the sulfur atom is bonded to the other two oxygen atoms through a single bond. These other two oxygen atoms hence carry a formal charge of -1 on each oxygen atom. These formal charges contribute to the overall charge (-2) of the sulfite anion. There is a lone electron pair on the sulfur atom. Therefore, the geometry of this anion is trigonal pyramidal.
What is Sulfur Trioxide?
Sulfur trioxide is an inorganic compound having the chemical formula SO3. It is considered the most important economic oxide compound of sulfur. This substance can exist in several forms: in the gas state, crystalline trimer state, and solid polymer. However, it is commercially available mainly as a colourless to white crystalline solid which can fume in air. The odour of this compound can vary, but it forms pungent vapour.
We can observe three resonance structures of sulfur trioxide compound. Therefore, the actual molecule is a hybrid structure of these three resonance structures. The hybrid structure has trigonal planar geometry. Here, the sulfur atom is in the center of the molecule, and it has +6 oxidation state. The formal charge on the sulfur atom is zero. The resonance structures indicate that the three sulfur to oxygen covalent bonds are equal in bond lengths.
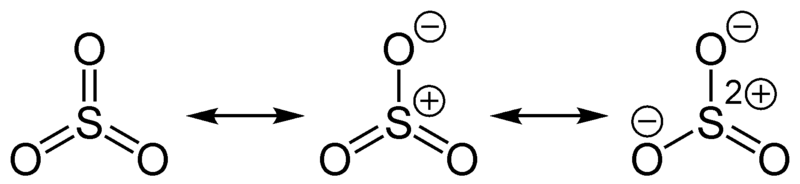
Figure 02: Resonance of Sulfur Trioxide Molecule
This material is important as a reagent in sulfonation reactions. These reactions are important in manufacturing detergents, dyes, and pharmaceutical compounds.
What is the Difference Between Sulfite and Sulfur Trioxide?
The term sulfites refer to the ionic compounds containing sulfite anion bound to different cations. Sulfur trioxide is an inorganic compound having the chemical formula SO3. The key difference between sulfite and sulfur trioxide is that sulfite is an ionic compound having a sulfate (IV) anion, whereas sulfur trioxide is a non-ionic compound.
The below infographic lists the differences between sulfite and sulfur trioxide in tabular form for side by side comparison.
Summary – Sulfite vs Sulfur Trioxide
The term sulfite refers to the ionic compounds containing sulfite anion bound to different cations. Sulfur trioxide is an inorganic compound having the chemical formula SO3. The key difference between sulfite and sulfur trioxide is that sulfites are ionic compounds having the sulfate (IV) anion, whereas sulfur trioxide is a non-ionic compound.
Reference:
1. “Sulfur Trioxide.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine.
Image Courtesy:
1. “Sulfite-ion-2D-dimensions” (Public Domain) via Commons Wikimedia
2. “SO3 meso” By Yikrazuul – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoysrKWemamybq3NnWSsrZybwrN506ugqLCZmbJw