The key difference between simple and complex coacervation is that in simple coacervation, only one type of polymer is used to make the coacervate, whereas, in complex coacervation, two or more polymers are used to make the coacervate.
Coacervation is the formation of a mixture of macromolecules such as synthetic polymers, proteins, or nucleic acids and an aqueous phase. This mixture forms through liquid-liquid phase separation. It leads to a dense phase that exists in thermodynamic equilibrium with a dilute phase. This mixture is called the coacervate. Moreover, dispersed droplets of the dense phase are also called coacervates.
We can name coacervates as lyophilic colloids. This means the dense phase retains some of the original solvent (e.g. water) and usually does not collapse to form solid aggregates; instead, it remains as a liquid property. There are two types as simple and complex coacervation that can be used to form a coacervate.
CONTENTS
1. Overview and Key Difference
2. What is Simple Coacervation
3. What is Complex Coacervation
4. Simple vs Complex Coacervation in Tabular Form
5. Summary – Simple vs Complex Coacervation
What is Simple Coacervation?
Simple coacervation is a technique that involves the use of a single polymer such as gelatin or ethyl cellulose for phase change. Therefore, simple coacervation needs only one type of polymer, whereas complex coacervation uses two or more types of polymers.
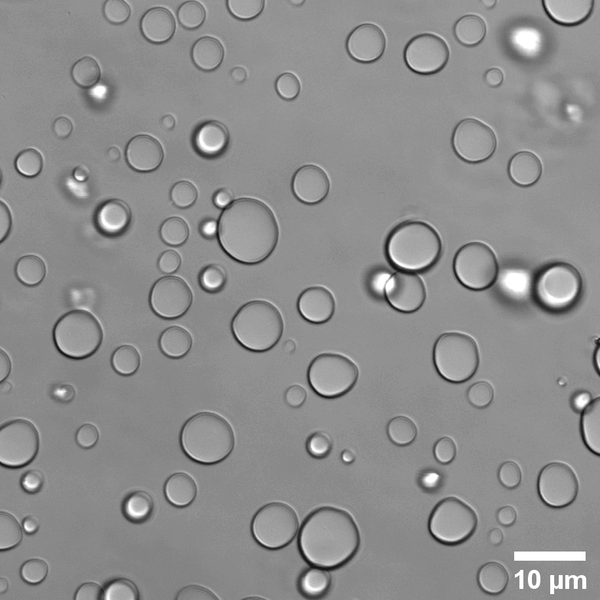
According to some research studies, simple coacervation can be triggered by certain salts. Typically, the phase separation in this process is brought about by the addition of a salt, pH, or temperature change in the polymeric solution.
What is Complex Coacervation?
Complex coacervation is a highly promising microencapsulation technique that is extensively useful in pharmaceutical, food, agricultural and textile industries. This process involves the interaction between oppositely charged polyelectrolytes in an aqueous form.
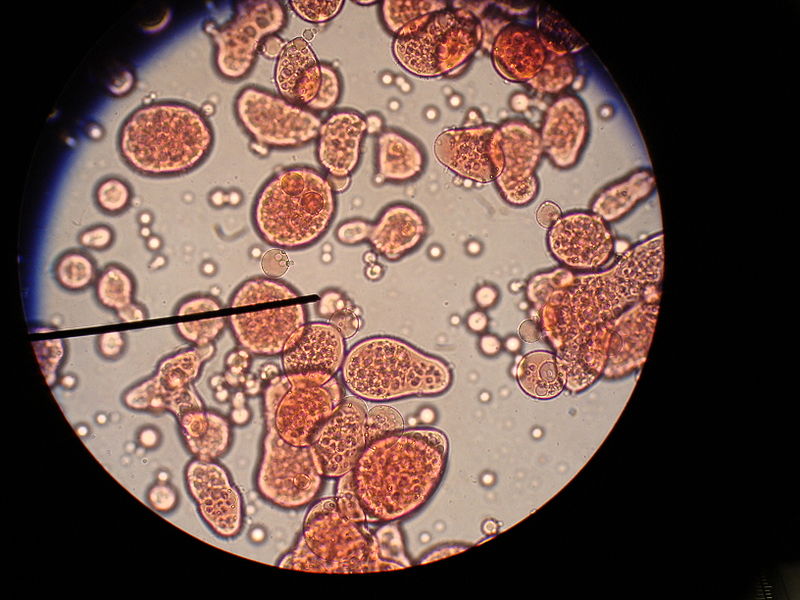
The complex coacervation method is the formaldehyde or glutaraldehyde-based technique. These techniques employ two natural biodegradable polymers of opposite charge. For example, we can use a pair such as an alginate and gelatin. Normally, gelatin is used as a cationic polymer. As the anionic polymer, there are various natural and synthetic water-soluble polymers that we can use to interact with gelatin to form a complex coacervate. However, the food industry typically uses gum Arabic exclusively.
What is the Difference Between Simple and Complex Coacervation?
Simple coacervation is a technique that involves the use of a single polymer such as gelatin or ethyl cellulose for phase change. Complex coacervation is a liquid-liquid phase separation occurring in solutions of oppositely-charged macromolecular species, like proteins, polymers, and colloids. The key difference between simple and complex coacervation is that in simple coacervation, only one type of polymer is used to make the coacervate, whereas, in complex coacervation, two or more polymers are used to make the coacervate.
Below is a summary of the difference between simple and complex coacervation in tabular form for side by side comparison.
Summary – Simple vs Complex Coacervation
Coacervation is the formation of a mixture of macromolecules such as synthetic polymers, proteins, or nucleic acids, and an aqueous phase. There are two types of conservation; they are simple and complex coacervation. The key difference between simple and complex coacervation is that simple coacervation uses only one type of polymer to make the coacervate, whereas complex coacervation uses two or more polymers.
Reference:
1. Astoricchio, Emanuele, et al. “The Wide World of Coacervates: From the Sea to Neurodegeneration.” Trends in Biochemical Sciences, vol. 45, no. 8, 2020, pp. 706–717., https://doi.org/10.1016/j.tibs.2020.04.006.
2. Manaf, M A, et al. “Encapsulation of Volatile Citronella Essential Oil by Coacervation: Efficiency and Release Study.” IOP Conference Series: Materials Science and Engineering, vol. 358, 2018, p. 012072., https://doi.org/10.1088/1757-899x/358/1/012072.
3. “Coacervation.” An Overview | ScienceDirect Topics.
Image Courtesy:
1. “Coacervate droplets example” By Spruijtlab – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Coacervats” By Khayman – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoysoKaonJp6orrDZpqopaChsrl5wqiYnJ2iq661tc6nZg%3D%3D