The key difference between diethyl ether and ethyl acetate is that diethyl ether has a rum-like odour, whereas ethyl acetate has an ether-like fruity odour.
Diethyl ether and ethyl acetate are important organic compounds. They have many applications in different industries and in laboratories, mainly as solvents.
CONTENTS
1. Overview and Key Difference
2. What is Diethyl Ether
3. What is Ethyl Acetate
4. Similarities – Diethyl Ether and Ethyl Acetate
5. Diethyl Ether vs Ethyl Acetate in Tabular Form
6. Summary – Diethyl Ether vs Ethyl Acetate
What is Diethyl Ether?
Diethyl ether is an organic compound with the chemical formula C2H5OC2H5. This substance is an ether with two ethyl groups attached to the same central oxygen atom. Diethyl ether is a colourless liquid that is highly volatile and flammable. Furthermore, it has a rum-like, sweet odour. This liquid is very useful as a solvent, a general anaesthetic, and a recreational drug due to its non-toxicity.
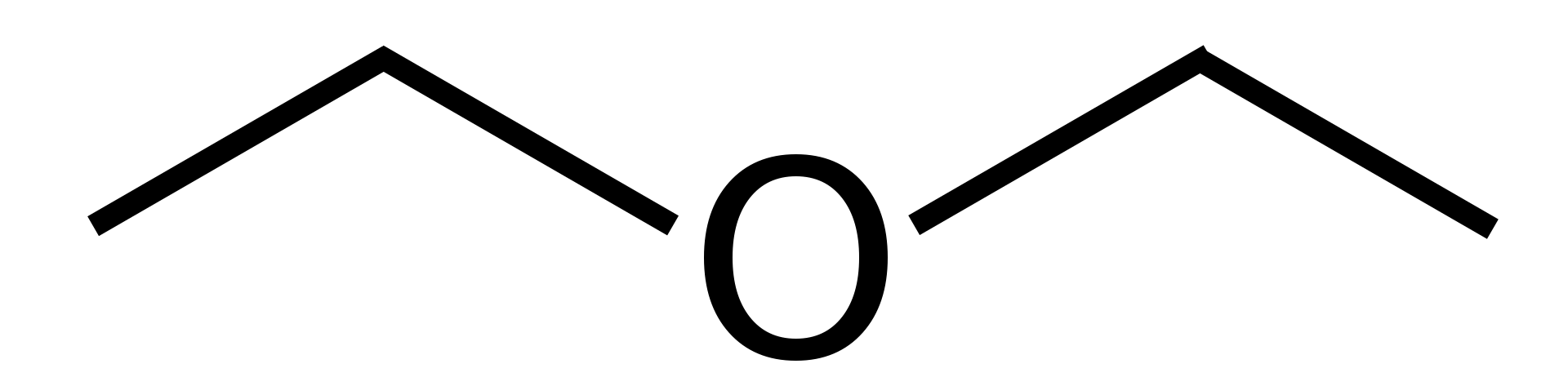
Figure 01: The Chemical Structure of Diethyl Ether
Diethyl ether is a functional group isomer of butanol. In other words, both diethyl ether and butanol have the same chemical formula, but diethyl ether has an ether functional group while butanol has an alcohol functional group.
When considering the production of diethyl ether, it is mostly produced as a byproduct of hydration of ethylene during the production of ethanol. Moreover, we can prepare diethyl ether via acid ether synthesis. In this process, we have to mix ethanol with strongly acidic sulfuric acid.
There are many useful applications of diethyl ether. For example, it is important as a solvent in laboratories, as a fuel or a starting fluid, as a general anaesthetic, and as a component in pharmaceutical formulations. However, despite the numerous uses of this compound, it is extremely volatile and flammable. This liquid is also sensitive to light and air; it tends to form explosive peroxides upon the explosion to light and air.
What is Ethyl Acetate?
Ethyl acetate is an organic compound with the chemical formula CH3CH2COOCH3. The molar mass of this substance is 88 g/mol. We can categorize this substance as a carboxylate ester because ethyl acetate forms from the interaction between a carboxylate group and an ethyl group, forming an ester bond. Moreover, Ethyl acetate is the ester of ethanol and acetic acid.
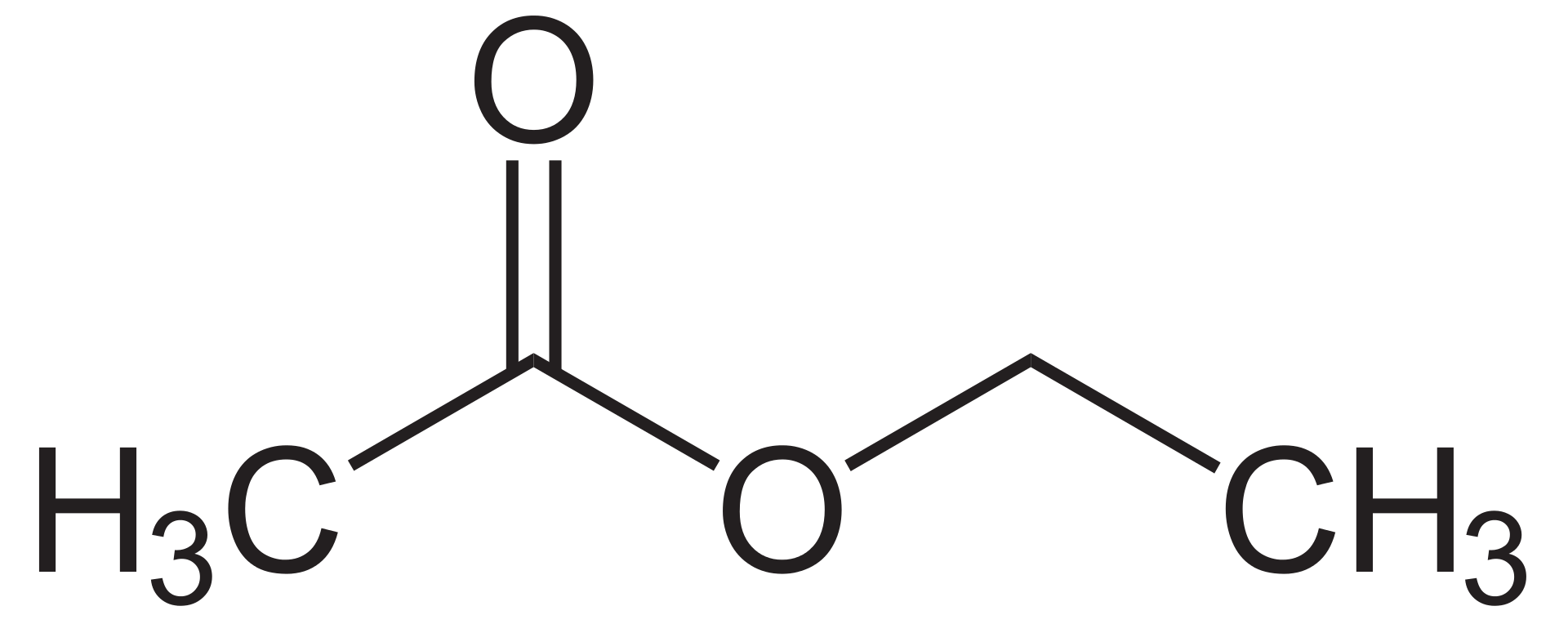
Figure 02: The Chemical Structure of Ethyl Acetate
At room temperature, ethyl acetate is a colourless liquid with a fruity odour. This liquid is also widely used as a solvent. Ethyl acetate vapour is heavier than normal air. There is a wide range of applications for this liquid because of its low cost, low toxicity, and pleasant odour.
The melting point of ethyl acetate is -83.6°C, while the boiling point is 77°C. It is a flammable liquid and is an irritant. Moreover, the hydrolysis of Ethyl acetate results in acetic acid and ethanol. This hydrolysis is a two-step process that occurs in the presence of a strong base such as sodium hydroxide (NaOH). The first step involves the formation of ethanol and sodium acetate, whereas the second step involves the conversion of sodium acetate into acetic acid.
What are the Similarities Between Diethyl Ether and Ethyl Acetate?
What is the Difference Between Diethyl Ether and Ethyl Acetate?
Diethyl ether is an organic compound with the chemical formula C2H5OC2H5 while ethyl acetate is an organic compound with the chemical formula CH3CH2COOCH3. The key difference between diethyl ether and ethyl acetate is that diethyl ether has a rum-like odour, whereas ethyl acetate has an ether-like fruity odour.
The below infographic presents the differences between diethyl ether and ethyl acetate in tabular form for side by side comparison.
Summary – Diethyl Ether vs Ethyl Acetate
Diethyl ether and ethyl acetate are important organic compounds. They have many applications in different industries and in laboratories, mainly as solvents. The key difference between diethyl ether and ethyl acetate is that diethyl ether has a rum-like odour, whereas ethyl acetate has an ether-like fruity odour.
Reference:
1. “Ethyl Acetate.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine.
Image Courtesy:
1. “Diethyl ether chemical structure” By Wolfmankurd at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
2. “Essigsäureethylester” By NEUROtiker (talk) – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoydoJ6smK65brHToZyrZZGjsW6x06GwpWWRmLK1rdOeZg%3D%3D