The key difference between dew point and freezing point is that dew point is the temperature at which the air becomes saturated with water vapor, whereas the freezing point is the temperature at which a liquid becomes solid.
Dew point and freezing point are important analytical parameters. These parameters or points represent the temperatures at which some changes to phase matter occurs.
CONTENTS
1. Overview and Key Difference
2. What is Dew Point
3. What is Freezing Point
4. Dew Point vs Freezing Point in Tabular Form
6. Summary – Dew Point vs Freezing Point
What is Dew Point?
Dewpoint temperature is the temperature at which the air becomes saturated with water vapor. In other words, it is the temperature to which we should cool the air to saturate the air with water vapor. Therefore, when further cooled, water vapor starts to condense and form dew drops. But when the temperature is below the freezing point of water, then we call the dewpoint “frost point” because at this temperature, frost, rather than dew, is formed.
When the dewpoint temperature equals the air temperature, it is the state of saturation of the air with water vapor. But this temperature never exceeds the air temperature. Therefore, if the air tends to cool down further, moisture is removed from the air via condensation.
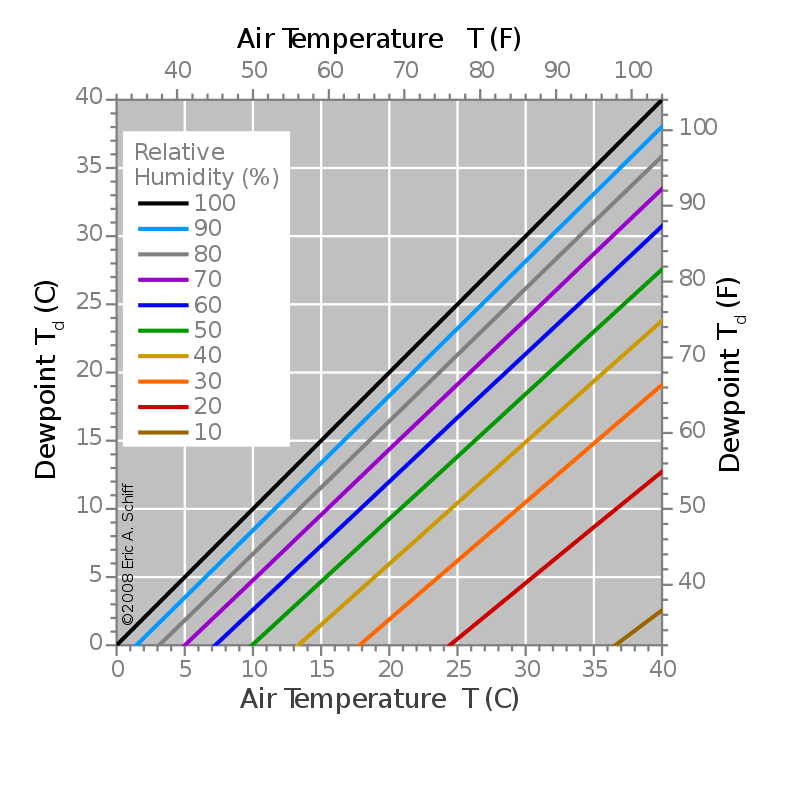
When considering the relationship between relative humidity and dewpoint;
- If the dewpoint is close to the dry air temperature, the relative humidity is high.
- If the dewpoint is well below the dry air temperature, the relative humidity is low.
What is Freezing Point?
The freezing point is the temperature at which a liquid becomes solid. At the freezing point, the liquid to the solid transition of the phase of matter occurs at the melting point, and the solid phase converts into its liquid phase. Theoretically, the freezing point is equal to the melting point. But practically, we can supercool liquids beyond the freezing point.
We can use the terms freezing and solidification interchangeably, but some tend to differentiate between these two terms because freezing occurs due to changes in temperature, while solidification can occur due to changes in pressure as well.

Knowing the freezing point of materials is very important in different applications. For example, in food preservation, where we can inhibit the decay of food and the growth of microorganisms, as well as freezing of living organisms or tissues during tissue preservation.
What is the Difference Between Dew Point and Freezing Point?
Dew point and freezing point are important analytical parameters. The key difference between dew point and freezing point is that dew point is the temperature at which the air becomes saturated with water vapor, whereas the freezing point is the temperature at which a liquid becomes solid.
The below infographic presents the differences between dew point and freezing point in tabular form for side by side comparison.
Summary – Dew Point vs Freezing Point
Dew point and freezing point are important analytical parameters. These parameters or points represent the temperatures at which some changes to phase matter occurs. The key difference between dew point and freezing point is that dew point is the temperature at which the air becomes saturated with water vapor, whereas the freezing point is the temperature at which a liquid becomes solid. Comparatively, the freezing point is greater in temperature than the dew point. It is an important fact in precipitation growth in clouds.
Reference:
1. “Dew Point vs Humidity.” NOAA’s National Weather Service, 26 Jan. 2021.
2. Lallanilla, Marc. “What Is Dew Point?” LiveScience, Purch, 11 Feb. 2014.
Image Courtesy:
1. “Dewpoint-RH” By Easchiff – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Freezing point depression and boiling point elevation” By User:Tomas erderivative work: InitHello (talk) – (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoydnLBloKS2r8CMmqWdZZansqbGyKeeZqifnru1ew%3D%3D