The key difference between xylitol and erythritol is that xylitol contains five carbon atoms and each carbon atom is attached to a hydroxyl group, whereas erythritol contains four carbon atoms, each carbon atom attached to a hydroxyl group.
Both xylitol and erythritol are sugar alcohols. These compounds are important as sugar substituents. Replacing sugar in food and drugs with xylitol or erythritol can promote better dental health and low blood sugar levels.
CONTENTS
1. Overview and Key Difference
2. What is Xylitol
3. What is Erythritol
4. Side by Side Comparison – Xylitol vs Erythritol in Tabular Form
5. Summary
What is Xylitol?
Xylitol is a chemical compound having the formula C2H12O5. It is a stereoisomer and occurs as a colorless or white crystalline solid that is water-soluble. We can classify this compound as a polyalcohol or sugar alcohol (an alditol). Xylitol is commonly used as a food additive to replace sugar in food and drugs. Therefore, we can name it as a sugar substituent.
Xylitol is a naturally occurring organic compound (in small amounts) in plums, strawberry, cauliflower, pumpkin, etc. Moreover, humans and many animals also make trace amounts of xylitol during the metabolism of carbohydrates. Xylitol is an achiral compound. That means, it has a plane of symmetry.
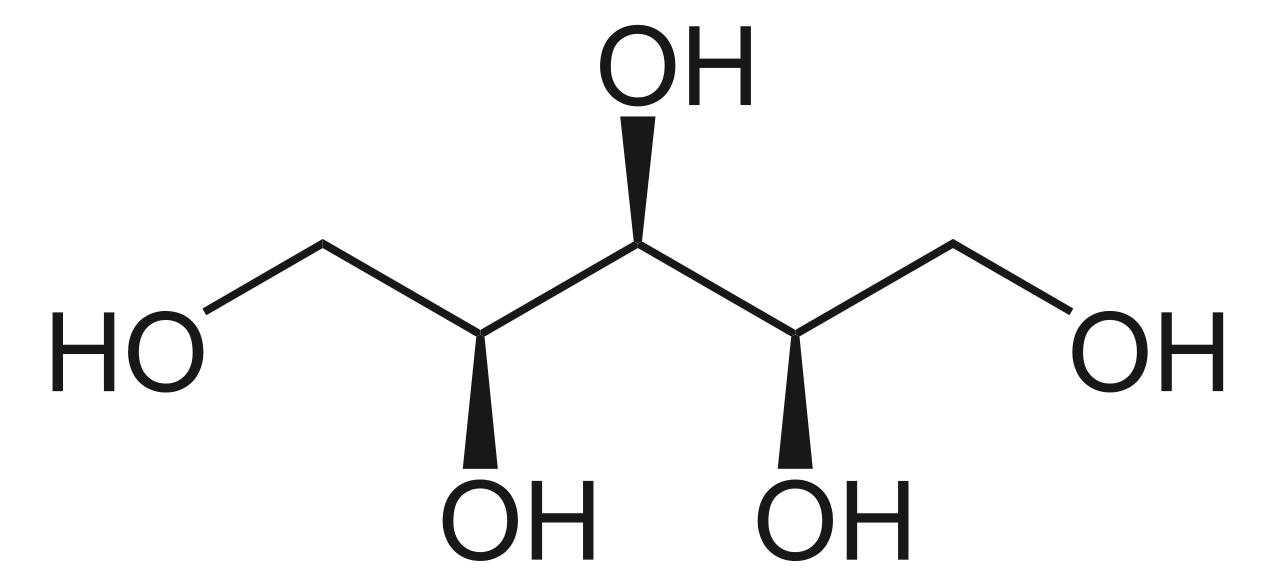
Figure 01: Xylitol
The industrial production of xylitol starts with lignocellulosic biomass from which xylan is extracted. The raw biomass material that can be used includes hardwood, softwood, agricultural waste from processing wheat, etc. Xylan is a polymer material that we can hydrolyze into xylose which is then catalytically hydrogenated into xylitol. This type of conversion changes the sugar xylose into the primary alcohol, xylitol.
There are many applications of xylitol as a sugar substituent. The products include drugs, dietary supplements, confections, toothpaste, chewing gum, etc. But it isn’t a common household sweetener. More importantly, this compound has a negligible effect on blood sugar level because xylitol undergoes metabolism independently of insulin.
What is Erythritol?
Erythritol is an organic compound having the chemical formula C4H10O4. It is a sugar alcohol, and we can use it as a food additive and as a sugar substituent. This compound is a naturally occurring substance, and we can make it from corn using enzymes and fermentation. It is a stereoisomer.
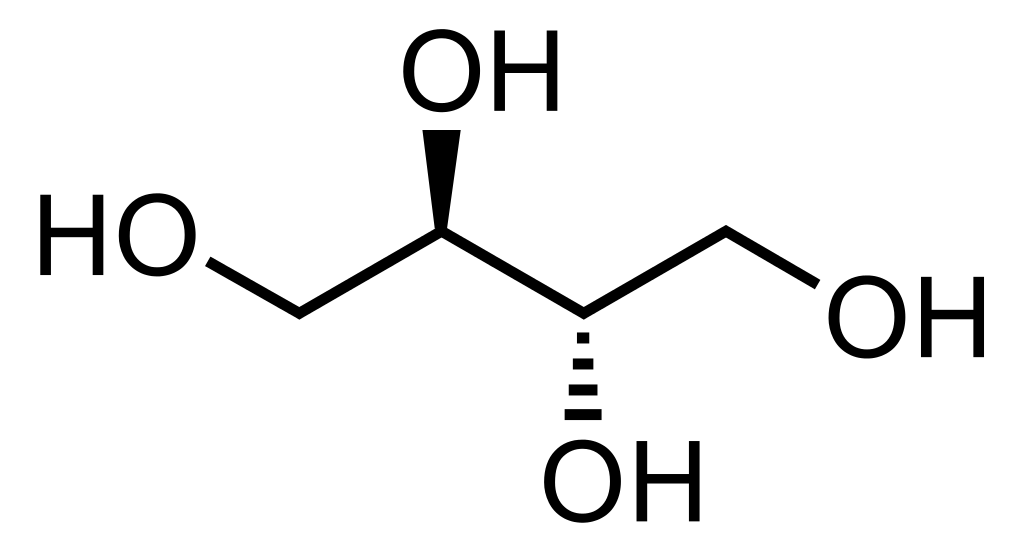
Figure 02: Erythritol
We can recognize erythritol 60-70% sweet as sucrose. However, it is almost noncaloric. Therefore, it does not affect the blood sugar level or causes tooth decay. Naturally, erythritol occurs in some fruits and fermented foods. Industrially, we can produce it from the fermentation of glucose with yeast.
There are many applications of erythritol as a food additive. Examples include beverages such as coffee, tea, liquid dietary supplements, juice blends, soft drinks, and flavored water.
When considering the production of erythritol, we can produce it from starch, beginning with enzymatic hydrolysis of starch obtained from corn to generate glucose. Then, glucose is fermented with yeast or another fungus to form erythritol.
What is the Difference Between Xylitol and Erythritol?
Both xylitol and erythritol are sugar alcohols. Xylitol is a chemical compound having the formula C2H12O5 while the erythritol is an organic compound having the chemical formula C4H10O4. The key difference between xylitol and erythritol is that xylitol contains five carbon atoms, whereas erythritol contains four carbon atoms.
The following infographic summarizes the differences between xylitol and erythritol in more detail.
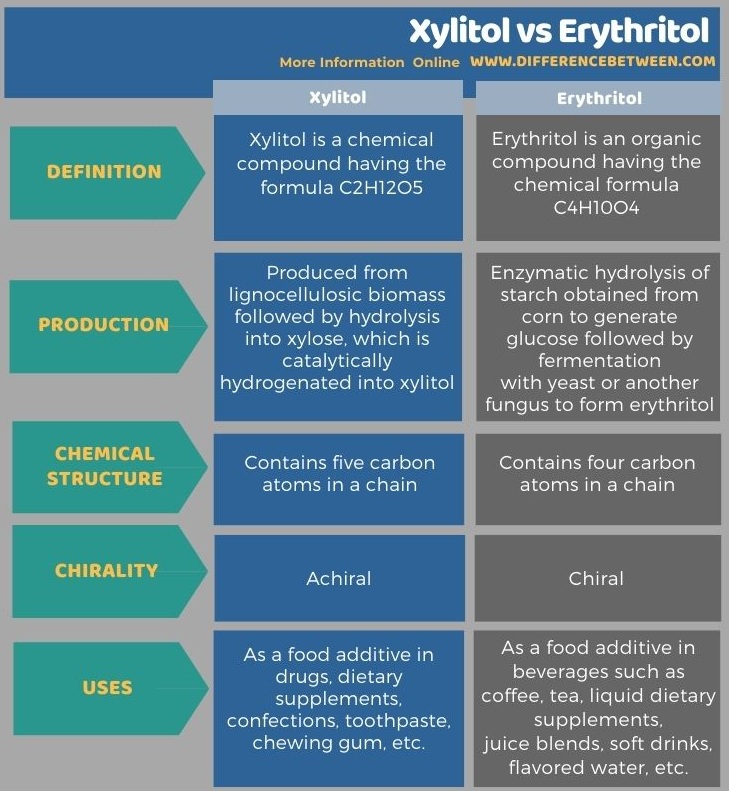
Summary – Xylitol vs Erythritol
Xylitol and erythritol are sugar alcohols that are important as sugar substitutes. The key difference between xylitol and erythritol is that xylitol contains five carbon atoms, each carbon atom attached to a hydroxyl group, whereas erythritol contains four carbon atoms, each carbon atom attached to a hydroxyl group.
Reference:
1. “Erythritol.” Wikipedia, Wikimedia Foundation, 4 Aug. 2020, Available here.
Image Courtesy:
1. “Xylitol-2D-structure” By Kemikungen – Own work (Public Domain) via Commons Wikimedia
2. “Erythritol structure” By Su-no-G – Own work made with ChemDraw (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27E2KWgraecYq6vsIyeqbKsmKe2tbvLaA%3D%3D