The key difference between xanthine and hypoxanthine is that xanthine is an oxidized form, whereas hypoxanthine is a reduced form.
Xanthine forms from the oxidation of hypoxanthine. Therefore, xanthine contains two carbonyl carbon atoms while hypoxanthine contains only one carbonyl carbon atom. Xanthine is an organic compound having the chemical formula C5H4N4O2. Both these are organic compounds containing double ring structures.
CONTENTS
1. Overview and Key Difference
2. What is Xanthine
3. What is Hypoxanthine
4. Side by Side Comparison – Xanthine vs Hypoxanthine in Tabular Form
5. Summary
What is Xanthine?
Xanthine is an organic compound having the chemical formula C5H4N4O2. It is a purine base. We can find this purine base in many human tissues and fluids. We can also find this compound in some other living organisms. This compound appears as a white solid, and it decomposes upon heating. The molar mass of xanthine is 152.11 g/mol.
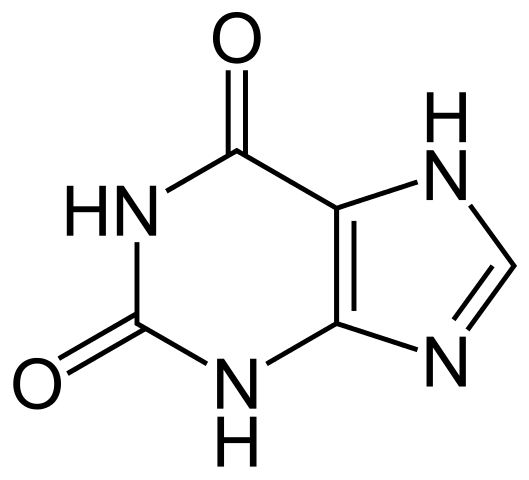
Figure 01: Chemical Structure of Xanthine
Xanthine forms as a product of the purine degradation pathway. There are three major reactions that can create xanthine; from guanine by guanine deaminase, from hypoxanthine by xanthine oxidoreductase, and from xanthosine by purine nucleoside phosphorylase.
There are many important uses of xanthine. For example, it is significant as a drug precursor for medication, as a pesticide ingredient, as a mild stimulant, as a bronchodilator, etc. However, this compound is known to be highly toxic in higher doses.
What is Hypoxanthine?
Hypoxanthine is the reduced form of xanthine. Therefore, we can call it a purine derivative. It naturally occurs, and we can find it as a component of nucleic acids. Hypoxanthine is the reduced form of xanthine. Xanthine forms upon the oxidation of hypoxanthine. Therefore, it has one carbonyl carbon, unlike xanthine (xanthine has two carbonyl carbon atoms). The IUPAC name if hypoxanthine is 1H-purin-6(9H)-one and the molar mass is 136.112 g/mol.

Figure 02: Chemical Structure of Hypoxanthine
Hypoxanthine is occasionally found in nucleic acid as a component. E.g. in the anticodon of tRNA. It exists there in the form of inosine. Hypoxanthine has a tautomer named 6-hydroxypurine. Moreover, it is an additive (in the form of a nitrogen source) in certain cells, bacteria, parasite cultures. Also, hypoxanthine is required in malaria parasite cultures as a source for nucleic acid synthesis and energy metabolism.
Hypoxanthine forms when xanthine oxidase enzyme acts on xanthine. It also forms as a product of spontaneous deamination of adenine. Adenine is similar to the structure of guanine. Therefore, this spontaneous deamination can lead to an error in DNA replication. Due to this reason, hypoxanthine is removed from DNA via base excision repair mechanisms.
What is the Difference Between Xanthine and Hypoxanthine?
The key difference between xanthine and hypoxanthine is that xanthine is an oxidized form, whereas hypoxanthine is a reduced form. Therefore, xanthine contains two carbonyl carbon atoms while hypoxanthine contains one carbonyl carbon atom. Moreover, the molar mass of xanthine is 152.11 g/mol, while the molar mass of hypoxanthine is 136.112 g/mol.
Furthermore, another difference between xanthine and hypoxanthine is that xanthine contains two oxygen atoms in its chemical structure, while hypoxanthine contains only one oxygen atom.
Below infographic summarizes the difference between xanthine and hypoxanthine.
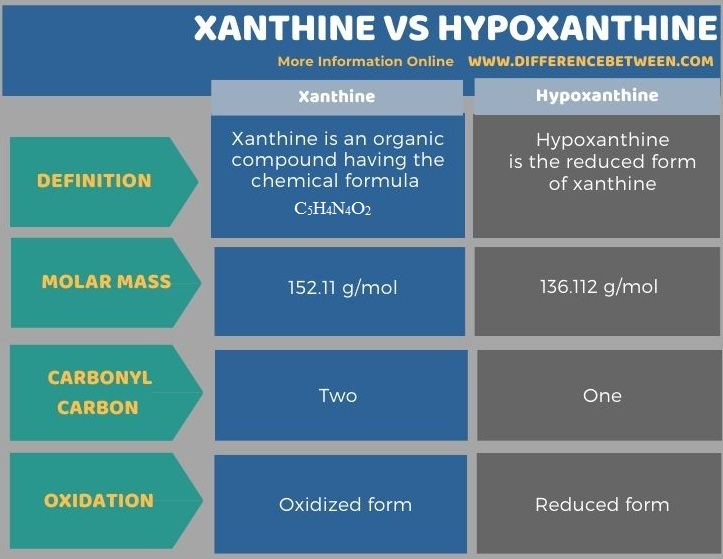
Summary – Xanthine vs Hypoxanthine
Xanthine and hypoxanthine are related to each other via chemical reactivity; xanthine forms upon the oxidation of hypoxanthine. The key difference between xanthine and hypoxanthine is that xanthine is an oxidized form, whereas hypoxanthine is a reduced form. Therefore, xanthine contains two carbonyl carbon atoms while hypoxanthine contains one carbonyl carbon atom.
Reference:
1. “Xanthine.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, Available here.
2. Helmenstine, Anne Marie. “The Difference Between Purines and Pyrimidines.” ThoughtCo, Feb. 11, 2020, Available here.
3. “Hypoxanthine.” Wikipedia, Wikimedia Foundation, 30 Dec. 2019, Available here.
Image Courtesy:
1. “Xanthin – Xanthine” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
2. “Hypoxanthin” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27EwKeroaGemnqiusNmn7Kon62ur8DHoqWeZw%3D%3D