The key difference between work and heat is that work is the ordered motion in one direction whereas heat is the random motion of molecules.
Work and heat are the two most important concepts of thermodynamics. Work and heat are highly interrelated to each other but they are not quite the same. The quest to understand work and heat goes way back. With these two concepts cleared, classical thermodynamics became one of the “completed” fields in physics. Both heat and work are concepts of energy. Theories of heat and work have a huge significance in thermodynamics, motor mechanics and machinery.
CONTENTS
1. Overview and Key Difference
2. What is Work
3. What is Heat
4. Side by Side Comparison – Work vs Heat in Tabular Form
5. Summary
What is Work?
In physics, we define work as the amount of energy transferred by a force acting through a distance. Work is a scalar quantity, which means there is only a magnitude to work, a direction is not present. Consider an object that we drag on a rough surface. There is friction acting on the object. For given points A and B, an infinite number of paths exist between them, therefore, there are infinitely many routes to take the box from A to B. If the distance the object travels when we take it on a certain path is, s, the work done by friction on the box is F.s, (considering only the scalar values). Different paths have different x values. Therefore, work done is different.
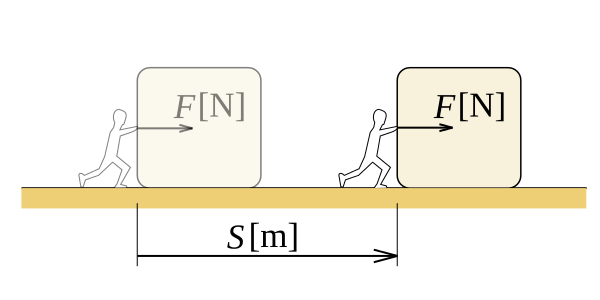
Figure 01: Work done during moving the object “s” distance with “F” Force
We can prove that the work depends on the path taken, which means work is a function of the path. For a conservative force field, we can take the work done as a function of the state. The SI unit of work is Joule, named in the honour of the English physicist James Joule. The CGS unit of work is erg. In thermodynamics, when we say work, we usually refer is to the pressure work, because the internal or external pressure is the force generator that does the work. In a constant pressure situation, the work done is P.ΔV, where P is the pressure and ΔV is the change in volume.
What is Heat?
Heat is a form of energy. We can measure it in Joule. The first law of thermodynamics is about the conservation of energy. It states that the heat supplied to a system is equal to the internal energy increment of that system plus the work done by the system on the surrounding. Thus, this shows that we can convert heat into work, and vice versa.

Figure 02: Fire produces Heat Energy
Furthermore, we can define the heat as the energy stored as random motion of molecules or atoms. The amount of heat in a system only depends on the state the system is in; therefore, heat is a function of state.
What is the Difference Between Work and Heat?
Work is the amount of energy transferred by a force acting through a distance while heat is a form of energy. The key difference between work and heat is that work is the ordered motion in one direction whereas heat is the random motion of molecules. Furthermore, work is a function of the path, but heat is a function of state.
As another important difference between work and heat, we can prove that work can be totally converted to heat, but heat cannot be 100% converted to work. Moreover, heat is a form of energy, while work is a method of transferring energy. The below infographic on difference between work and heat gives a more detailed comparison.
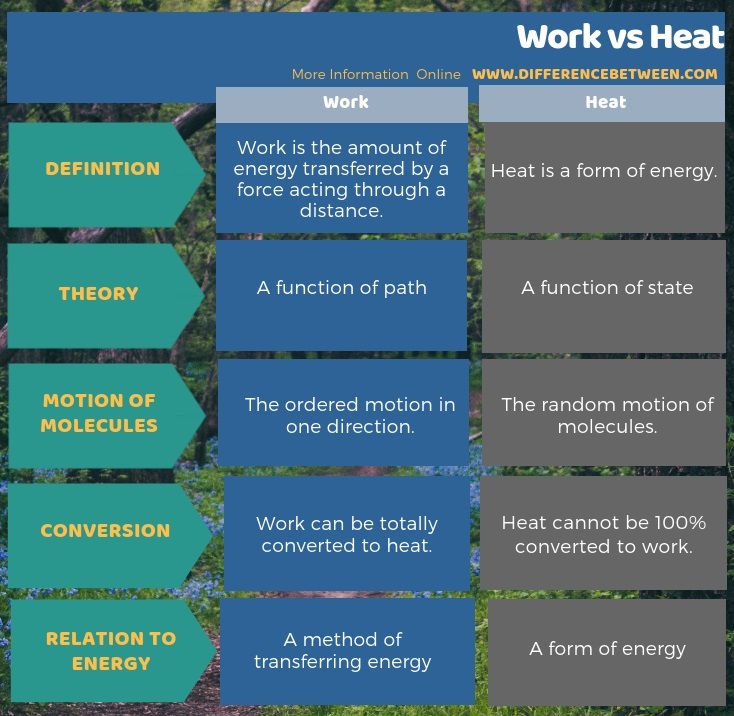
Summary – Work vs Heat
Work and heat are concepts that we use in both physics and chemistry. Work and heat are interrelated however there are some differences between them as well. The key difference between work and heat is that work is the ordered motion in one direction whereas heat is the random motion of molecules.
Reference:
1. OpenStaxCollege. “College Physics.” Introduction to Sociology – 1st Canadian Edition, BCcampus, 23 Jan. 2012. Available here
2. Jones, Andrew Zimmerman. “A Scientific Way to Define Heat Energy.” ThoughtCo, Oct. 11, 2018. Available here
Image Courtesy:
1.”Work (physics)”By すじにくシチュー – Own work, (CC0) via Commons Wikimedia
2.”624524″ (CC0) via pxhere
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27DzquiZpmemXq3v4yhnJqsXw%3D%3D