The key difference between ubiquitination and sumoylation is that ubiquitination is a post-translational modification which can mark proteins for degradation or have other singling functions while sumoylation is a post-translational modification which is not used in cells to mark proteins for degradation.
Post-translational modifications are covalent and enzymatic modifications that happen after protein synthesis. These modifications regulate protein activities. Attachment of small chemical groups, sugar, lipids and polypeptides modify proteins. Ubiquitin is the most well-known polypeptide modifier. Moreover, there are several ubiquitin-like proteins. Small ubiquitin-related modifier (SUMO) is one such modifier. Therefore, ubiquitination and sumoylation are two post-translational modifications. Ubiquitination marks proteins for degradation. In contrast, sumoylation is not used to mark proteins for degradation. Both modifications regulate protein localization and activity. They are reversible processes.
CONTENTS
1. Overview and Key Difference
2. What is Ubiquitination
3. What is SUMOylation
4. Similarities Between Ubiquitination and SUMOylation
5. Side by Side Comparison – Ubiquitination vs SUMOylation in Tabular Form
6. Summary
What is Ubiquitination?
Ubiquitin is a polypeptide modifier. It is a small protein acting as a molecular tag in post-translational modification of proteins. Ubiquitination is the process that uses ubiquitin for post-translational modification. Different enzymes catalyze the covalent conjugation of ubiquitin to proteins. It takes place in the presence of ATP. The enzymes that catalyze ubiquitination are ubiquitin-activating enzymes, ubiquitin-conjugating enzymes and ubiquitin ligases.
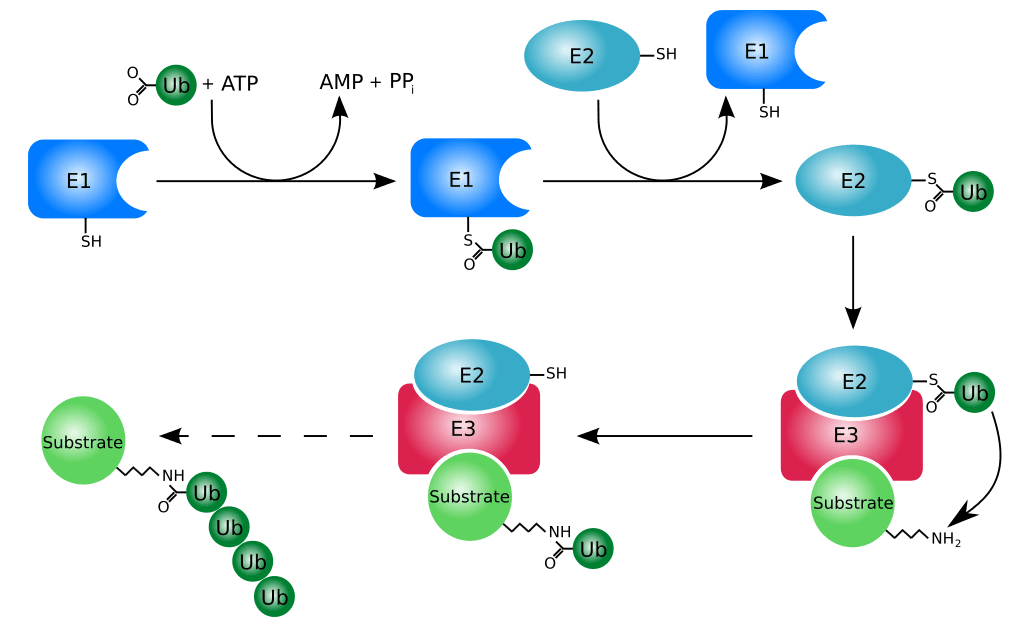
Figure 01: Ubiquitination
Ubiquitination plays an important role in targeting proteins for proteolytic degradation. Moreover, ubiquitination can also regulate protein localization and activity. This process can be reversed through the action of deubiquitinase enzymes.
What is SUMOylation?
SUMOylation is another post-translational modification using small ubiquitin-like modifiers (SUMOs). Covalent attachment of SUMOs alters the structure and function of the protein. SUMOylation covalently modifies a large number of proteins involved in many cellular processes including gene expression, chromatin structure, signal transduction, and maintenance of the genome.
Figure 02: SUMO Protein
SUMOylation regulates protein localization and activity similar to ubiquitination. However, unlike ubiquitination, sumoylation does not tag or mark proteins for degradation. Similar to ubiquitination, sumoylation is an enzymatic process which is catalyzed by enzymes.
What are the Similarities Between Ubiquitination and SUMOylation?
- Ubiquitination and Sumoylation are two important post-translational modifications.
- Both sumoylation and ubiquitination are reversible processes.
- They play crucial roles in biological functions.
- Some proteins can be modified by SUMO and ubiquitin.
- Both processes alter protein function.
- Moreover, both post-translational modifications regulate protein localization and activity.
- They require a cascade of enzymes.
What is the Difference Between Ubiquitination and SUMOylation?
Ubiquitination and SUMOylation are two post-translational modifications which alter the function of proteins. Ubiquitination is the covalent conjugation of ubiquitin to proteins while SUMOylation is the addition of SUMOs to proteins. Moreover, ubiquitination tags proteins for proteolytic degradation while SUMOylation does not tag proteins for degradation. Thus, this is the key difference between ubiquitination and sumoylation.
Below infographic shows more details of the difference between ubiquitination and sumoylation.
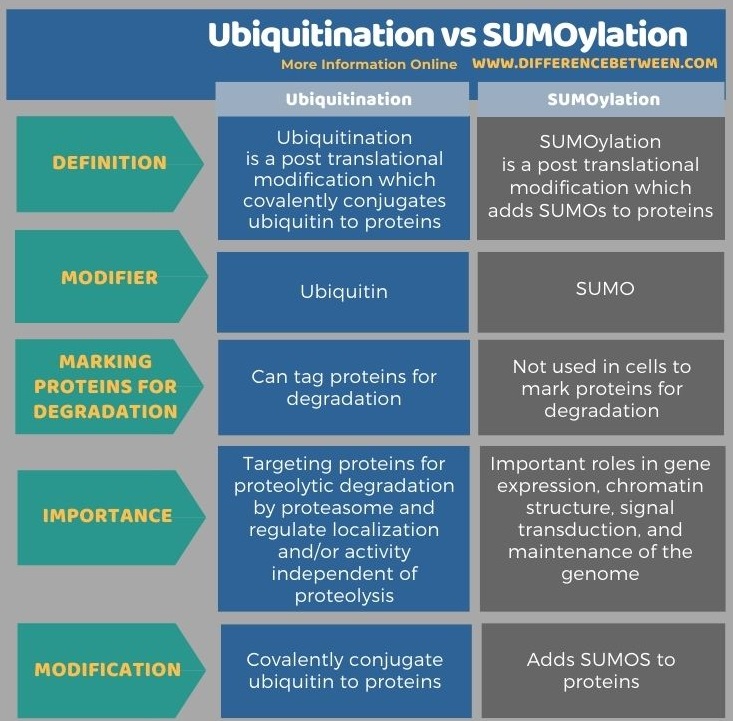
Summary – Ubiquitination vs SUMOylation
Ubiquitination and sumoylation are two important post-translational modifications. Both are reversible processes catalyzed by enzymes. In ubiquitination, ubiquitin is the polypeptide modifier while in sumoylation, SUMOs are the modifiers. Ubiquitins are covalently conjugated with proteins and alter their structure and function. SUMOs are added to proteins in sumoylation. SUMOylation is analogous to ubiquitylation in terms of the reaction scheme and enzyme classes used. But, ubiquitination tags proteins for proteasome-dependent degradation while sumoylation does not involve in tagging proteins for degradation. Thus, this is the key difference between ubiquitination and sumoylation.
Reference:
1. Gill, Grace. “SUMO and Ubiquitin in the Nucleus: Different Functions, Similar Mechanisms?” Genes & Development, Cold Spring Harbor Lab, 1 Jan. 1970, Available here.
2. Smith, Yolanda. “Ubiquitination (Ubiquitylation).” News, 23 Aug. 2018, Available here.
Image Courtesy:
1. “Ubiquitylation” By Rogerdodd (CC BY-SA 3.0) via Commons Wikimedia
2. “HSUMO1 1A5R nmr ribbons” By Jakob Suckale at English Wikipedia – Originally from en.wikipedia (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27BwaKorqGknruiwMiopWaZnpl6tMHMqLClmaSevK97