The key difference between true and pseudo critical properties is that the true critical properties are the real critical properties of a system, which are determined thermodynamically, whereas the pseudo critical properties are the apparent contribution of each pure component in a system to a particular reaction.
The term critical properties refer to the temperature and pressure of a system at the critical point. The critical point of a thermodynamic system is the endpoint of the phase equilibrium curve of that system. It is the temperature and pressure at which a liquid can coexist with its vapour phase. Generally, properties that we consider as critical properties are critical temperature and critical pressure.
CONTENTS
1. Overview and Key Difference
2. What are True Critical Properties
3. What are Pseudo Critical Properties
4. Side by Side Comparison – True vs Pseudo Critical Properties in Tabular Form
5. Summary
What are True Critical Properties?
True critical properties are the real critical properties of a system that are determined thermodynamically. The true critical properties are realistic values because they are calculated thermodynamically. And in calculating these values, they satisfy both quadratic and cubic forms in the expansion of the Helmholtz free energy as a function of the mole numbers as zero at a critical point.
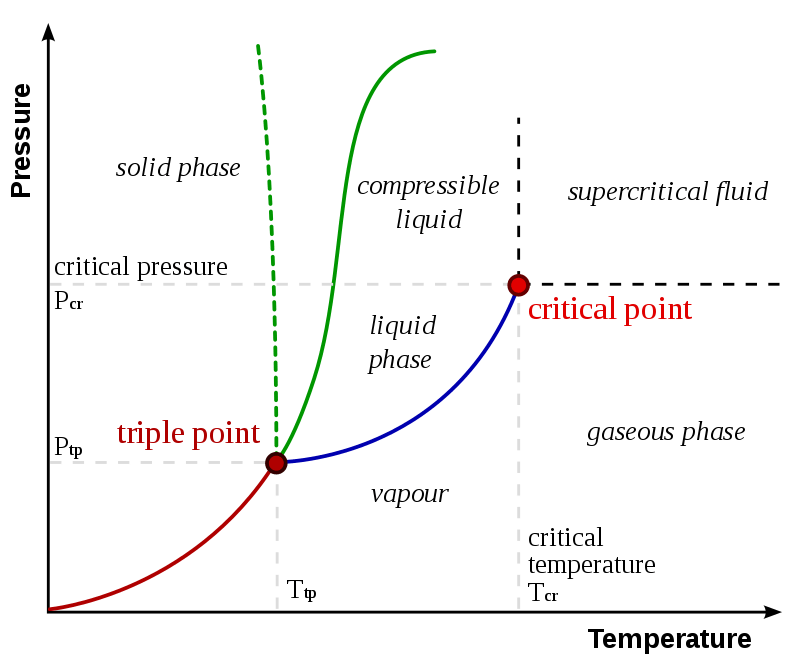
Figure 01: A Phase Diagram Showing the Critical Point
What are Pseudo Critical Properties?
Pseudo critical properties are the properties of a system that are apparent contributions of each pure component in a system to a particular reaction. These values are calculated for mixtures such as gas mixtures. These properties are also named as scaling factors. Furthermore, we can describe these pseudo critical properties as characterizing constants that are usually obtained through a process which involves a process of averaging the constants of the pure constituent components in a mixture; especially, gaseous mixtures. Therefore, the pseudo critical properties differ from the true critical properties substantially.
What is the Difference Between True and Pseudo Critical Properties?
The term critical properties usually refer to the temperature and pressure of a system at the critical point. There are two types of critical properties; they are true critical properties and pseudo critical properties. The key difference between true and pseudo critical properties is that the true critical properties are the real critical properties of a system that are determined thermodynamically whereas the pseudo critical properties are the apparent contribution of each pure component in a system to a particular reaction.
Moreover, the true critical properties are calculated using thermodynamic processes, while the pseudo critical properties are calculated using linear models. Besides, true critical properties give realistic values while pseudo critical properties give apparent values. Thus, this is another difference between true and pseudo critical properties.
The following infographic summarizes the difference between true and pseudo critical properties in tabular form.
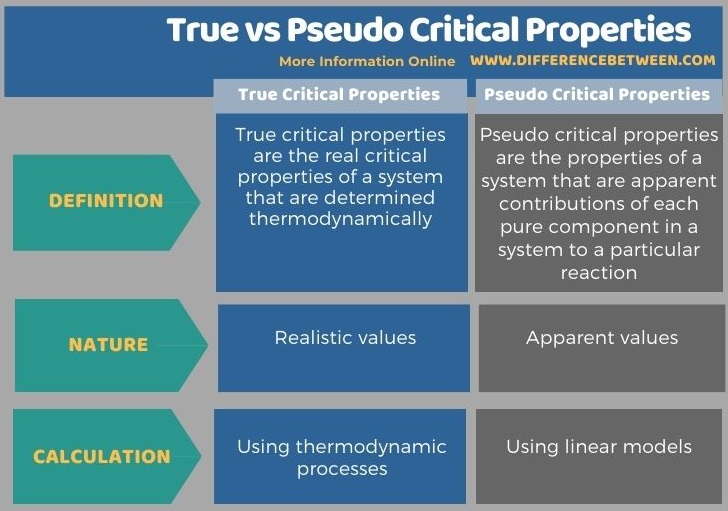
Summary – True vs Pseudo Critical Properties
The term critical properties usually refer to the temperature and pressure of a system at the critical point. True critical properties are the real critical properties of a system that are determined thermodynamically. Pseudo critical properties, on the other hand, are the apparent contribution of each pure component in a system to a particular reaction. Thus, this is the key difference between true and pseudo critical properties. Therefore, true critical properties give realistic values while pseudo critical properties give apparent values.
Reference:
1. “Critical Point.” Chemistry | Libretexts. MindTouch, 15 Aug. 2020. Web. Available here.
2. “True Or Pseudo Critical Pressure For Sizing Control Valves – Chemical Process Simulation.” Cheresources.com Community. 13 Nov. 2013. Web. Available here.
3. “Pseudocritical Pressure – an Overview.” ScienceDirect Topics. Web. Available here.
4. Fenghour, A. “Critical Properties of Mixtures.” Heat Exchanger Design Handbook. Web. Available here.
Image Courtesy:
1. “Phase-diag2” By Matthieumarechal (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27A0a6cZpmemXqxv8Sum6hlk6e2tbXCmqNmqKKkvaa%2B06KcrGc%3D