The key difference between transuranic elements and radioisotopes is that transuranic elements are the chemical elements having atomic numbers greater than 92, whereas radioisotopes are unstable atoms which are radioactive.
Both transuranic elements and radioisotopes are radioactive chemical elements. Most of the times, radioactive atoms have a high atomic number, but sometimes there can be rare isotopes of some chemical elements with a small atomic number, which are radioactive due to an imbalance of protons and neutrons in their nuclei.
CONTENTS
1. Overview and Key Difference
2. What are Transuranic Elements
3. What are Radioisotopes
4. Side by Side Comparison – Transuranic Elements vs Radioisotopes in Tabular Form
5. Summary
What are Transuranic Elements?
Transuranic elements or transuranium elements are chemical elements having atomic numbers higher than 92. The atomic number of Uranium is 92; therefore, the series of transuranic elements starts with Uranium, which leads the name of this series (trans + uranium). All the members of this list are radioactive due to their unstable nature.
Most of the chemical elements in the periodic table have isotopes we can find in the universe as either stable atoms or as chemical elements having a very long half-life. These chemical elements are in the range of 1 to 92 atomic numbers.
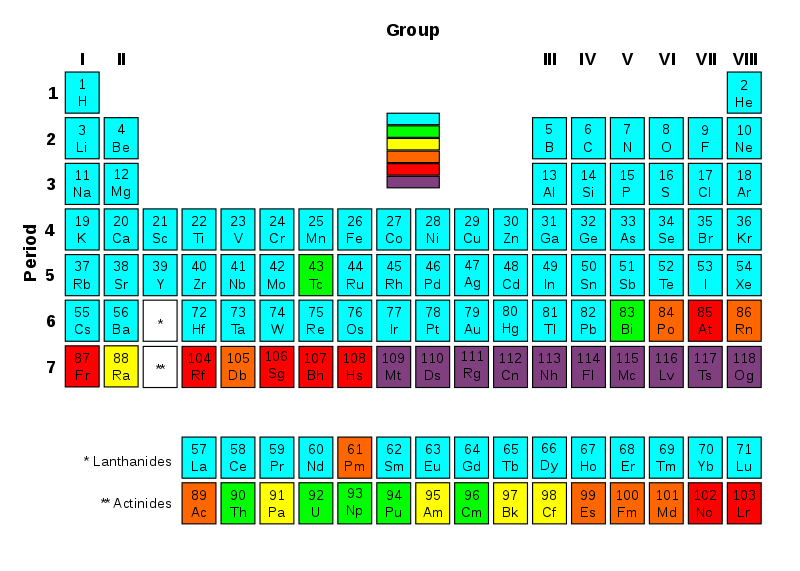
Figure 01: Transuranic Elements
We can generate transuranic elements via using synthetic elements, using nuclear reactors or using particle accelerators. There is a relationship between the atomic number and the half-life of these elements. The half-lives generally decrease with increasing atomic numbers. However, there can be some exceptions due to some isotopes; for example, isotopes of Curium and Dubnium.
List of Transuranic Elements
- Actinides
- Neptunium
- Plutonium
- Americium
- Curium
- Berkelium
- Californium
- Einsteinium
- Fermium
- Mendelevium
- Nobelium
- Lawrencium
- Transactinide elements
- Rutherfordium
- Dubnium
- Seaborgium
- Bohrium
- Hassium
- Meitnerium
- Darmstadtium
- Roentgenium
- Copernicium
- Nihonium
- Flerovium
- Moscovium
- Livermorium
- Tennessine
- Oganesson
- Elements in period 8 (not discovered yet)
What are Radioisotopes?
Radioisotopes are radioactive isotopes of chemical elements. These isotopes are unstable because they have excess nuclear energy. There are three ways that a radioisotope releases this nuclear energy:
If one of the above three actions occur, we say that radioactive decay has taken place. We name these emissions as ionizing radiation because these emitted rays can ionize another atom to liberate an electron.

Figure 02: Americium is a Radioisotope
All chemical elements may exist as radioactive atoms in their isotopic forms. For example, even the lightest element hydrogen has a radioactive isotope – tritium. Moreover, some chemical elements exist only as radioactive elements.
What is the Difference Between Transuranic Elements and Radioisotopes?
Both transuranic elements and radioisotopes are radioactive chemical elements. The key difference between transuranic elements and radioisotopes is that the transuranic elements are the chemical elements having atomic numbers greater than 92, whereas the radioisotopes are unstable atoms which are radioactive.
Moreover, the transuranic elements exist only as radioactive atoms, while the radioisotopes are isotopes of chemical elements that exist as radioactive atoms. For example, actinide series, transactinide series and elements of period 8 are transuranic elements. Tritium isotope of hydrogen is a very light radioisotope with a very low atomic number.
Below infographic summarizes the difference between transuranic elements and radioisotopes.
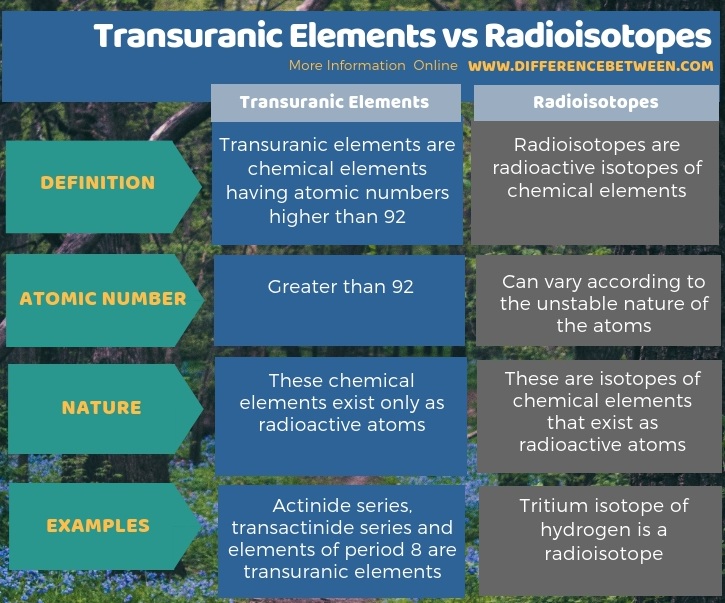
Summary – Transuranic Elements vs Radioisotopes
Both transuranic elements and radioisotopes are radioactive chemical elements. The key difference between transuranic elements and radioisotopes is that the transuranic elements are the chemical elements having atomic numbers greater than 92, whereas the radioisotopes are unstable atoms which are radioactive.
Reference:
1. Fry, Brian. “Using Stable Isotope Tracers.” Stable Isotope Ecology, 2006, pp. 40–75., doi:10.1007/0-387-33745-8_3.
2. “Radioisotopes.” IAEA, IAEA, 15 July 2016, Available here.
Image Courtesy:
1. “Periodic Table Radioactivity” By Periodic_Table_Armtuk3.svg: Armtuk (talk)derivative work: Alessio Rolleri (talk)derivative work: Gringer (talk) – Periodic_Table_Armtuk3.svg (CC BY-SA 3.0) via Commons Wikimedia
2. “Americium-241 Sample from Smoke Detector” By MedicalReference – Self-photographed (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27A0ZqlrK2ilruqr4yeo56llaPBtHnAp5tmqpGZtrC10qirqKiVqHw%3D