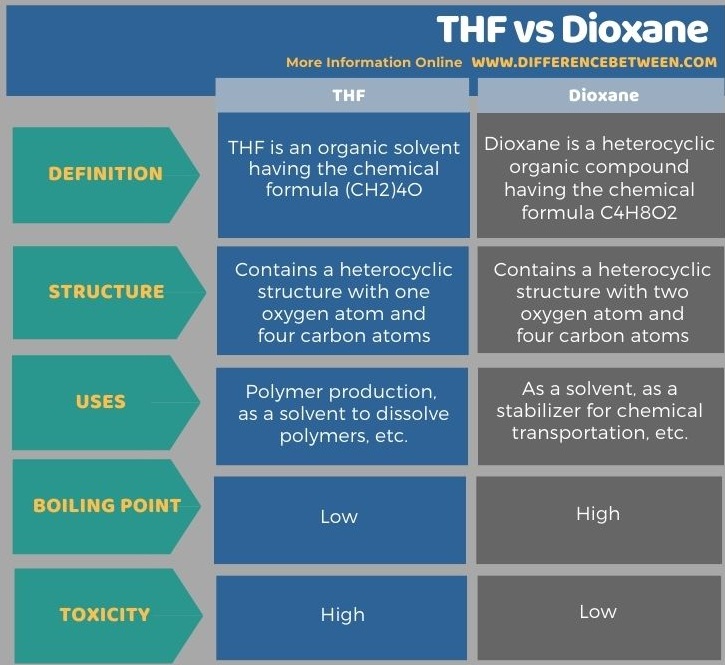
The key difference between THF and dioxane is that THF molecules contain one oxygen atom as a member of the ring structure whereas dioxane molecule contains two oxygen atoms as members of the ring structure.
Both THF and dioxane are organic solvents that are important in analyzing samples. These organic structures are cyclic structures that we can classify as heterocyclic compounds because these ring structures contain two types of atoms forming the ring: carbon atoms and oxygen atom(s).
CONTENTS
1. Overview and Key Difference
2. What is THF
3. What is Dioxane
4. Side by Side Comparison – THF vs Dioxane in Tabular Form
5. Summary
What is THF?
THF is an organic solvent having the chemical formula (CH2)4O. It is a heterocyclic compound, and we can categorize it as an ether because the functional group of the THF molecule is –C-O-C-. We can observe THF as a colourless organic liquid which is miscible with water. This solvent has an ether-like odour. It has a low viscosity as well. This solvent is mainly used as a precursor for polymer synthesis processes. THF is a polar molecule which is helpful in mixing it with water. In addition to these, this polarity makes THF a versatile solvent.

Figure 01: THF Solvent
When considering the applications of THF solvent, it is important in polymerization processes; in the presence of strong acids, THF converts into a linear polymer material, poly(tetramethylene ether) glycol or PTMEG. This polymer material is useful in the production of elastomeric polyurethane fibres such as spandex.
Furthermore, THF is important as a solvent for PVC and in varnishes. This is because THF is an aprotic solvent having a dielectric constant of 7.6. We can classify THF as a moderately polar solvent which can dissolve a wide range of nonpolar and polar chemical compounds.
Moreover, THF is useful as a component in the mobile phase for reversed-phase liquid chromatography. THF is used in this technique because it has a great elution strength than methanol or acetonitrile, but less commonly used than these solvents.
What is Dioxane?
Dioxane is a heterocyclic organic compound having the chemical formula C4H8O2. We can classify it as an ether where there are two –C-O-C- ether groups. It exists as a colourless liquid having a mild ether-like odour. There are three isomers of dioxane as 1,2-dioxane, 1,3—dioxane, and 1,4-dioxane. Among these three compounds, 1,4-dioxane is the common compound where other compounds are rarely encountered.
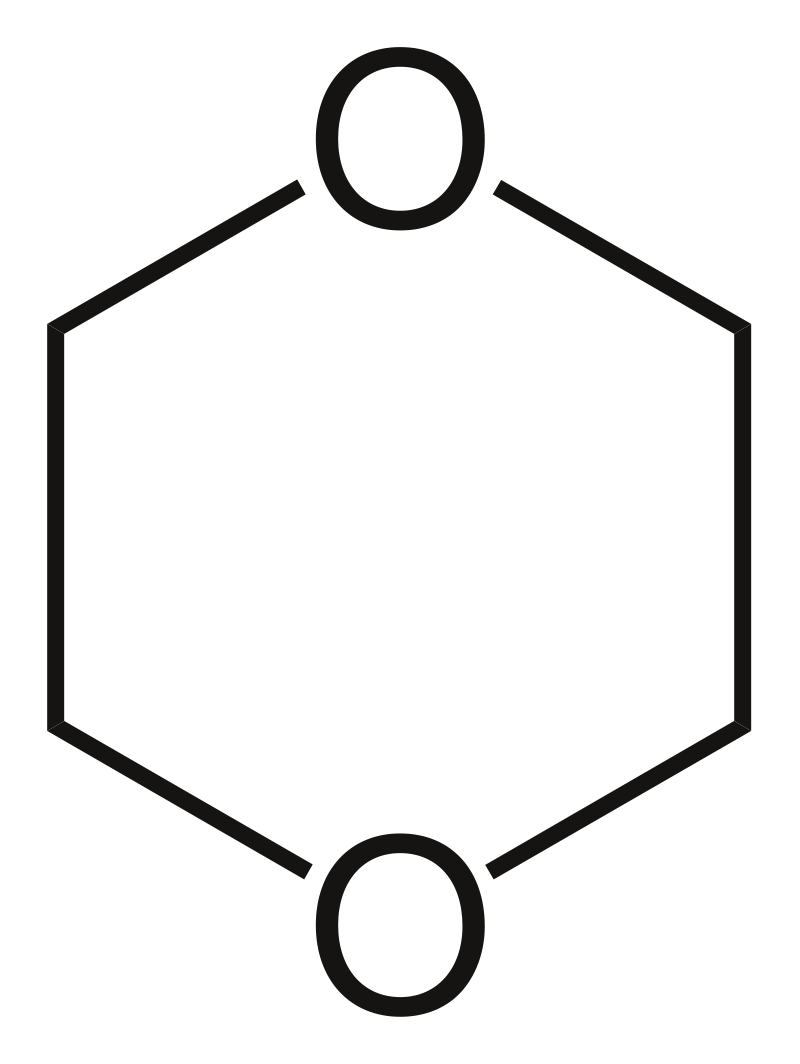
Figure 02: Structure of Dioxane Molecule
When considering the synthesis, dioxane can be produced via the acid-catalyzed dehydration of diethylene glycol. We can obtain diethylene glycol from the hydrolysis of ethylene oxide. This liquid is miscible with water because it is polar.
Dioxane is important in trichloroethane transport as a stabilizer. Moreover, it is important as an aprotic solvent for inks, adhesives, and cellulose esters. We can use this solvent as a substitute from THF in some processes due to the low toxicity and high boiling point of dioxane solvent.
What is the Difference Between THF and Dioxane?
Both THF and dioxane are organic solvents that are important in analyzing samples. The key difference between THF and dioxane is that THF molecules contain one oxygen atom as a member of the ring structure whereas dioxane molecule contains two oxygen atoms as members of the ring structure. We can use dioxane as a substitute for THF due to low toxicity and high boiling point.
Below infographic shows more details of the difference between THF and dioxane.
Summary – THF vs Dioxane
Both THF and dioxane are organic solvents that are important in analyzing samples. The key difference between THF and dioxane is that THF molecules contain one oxygen atom as a member of the ring structure whereas dioxane molecule contains two oxygen atoms as members of the ring structure.
Reference:
1. “1,4-Dioxane.” Wikipedia, Wikimedia Foundation, 9 Sept. 2020, Available here.
Image Courtesy:
1. “Tetrahydrofuran sample” By LHcheM – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “1-4-Dioxane” By Rhododendronbusch – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27Ax59kmqaUYrGqu9eapZ5n