Key Difference – Strong Ligand vs Weak Ligand
A ligand is an atom, ion, or a molecule that donates or shares two of its electrons through a coordinate covalent bond with a central atom or ion. The concept of ligands is discussed under coordination chemistry. Ligands are chemical species that are involved in the formation of complexes with metal ions. Hence, they are also known as complexing agents. Ligands can be Monodentate, bidentate, tridentate, etc. based on the denticity of the ligand. Denticity is the number of donor groups present in a ligand. Monodentate means that ligand has only one donor group. Bidentate means it has two donor groups per one ligand molecule. There are two major types of ligands categorized based on crystal field theory; strong ligands (or strong field ligands) and weak ligands (or weak field ligands). The key difference between strong ligands and weak ligands is that the splitting of orbitals after binding to a strong field ligand causes a higher difference between the higher and lower energy level orbitals whereas the splitting of orbitals after binding to a weak field ligand causes a lower difference between the higher and lower energy level orbitals.
CONTENTS
1. Overview and Key Difference
2. What is Crystal Field theory
3. What is Strong Ligand
4. What is Weak Ligand
5. Side by Side Comparison – Strong Ligand vs Weak Ligand in Tabular Form
6. Summary
What is Crystal Field Theory?
Crystals field theory can be described as a model that is designed to explain the breaking of degeneracies (electron shells of equal energy) of electron orbitals (usually d or f orbitals) due to the static electric field produced by a surrounding anion or anions (or ligands). This theory is often used to demonstrate the behaviour of transition metal ions complexes. This theory can explain the magnetic properties, colours of coordination complexes, hydration enthalpies, etc.
Theory:
The interaction between the metal ion and ligands is a result of the attraction between the metal ion with a positive charge and the negative charge of the unpaired electrons of the ligand. This theory is mainly based on the changes occuring in five degenerated electron orbitals (a metal atom has five d orbitals). When a ligand come close to the metal ion, the unpaired electrons are closer to some d orbitals than that of other d orbitals of the metal ion. This cause a loss of degeneracy. And also, the electrons in the d orbitals repel the electrons of the ligand (because both are negative charged). Hence the d orbitals that are closer to the ligand has high energy than that of other d orbitals. This result in the splitting of d orbitals into high energy d orbitals and low energy d orbitals, based on the energy.
Some factors affecting this splitting are; nature of the metal ion, the oxidation state of metal ion, the arrangement of ligands around the central metal ion and the nature of ligands. After the splitting of these d orbitals based on energy, the difference between the high and low energy d orbitals is known as a crystal-field splitting parameter (∆oct for octahedral complexes).
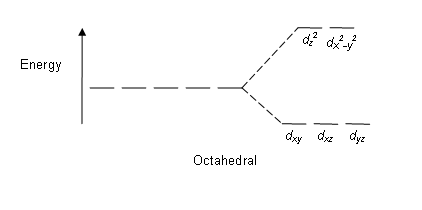
Figure 01: Splitting Pattern in Octahedral Complexes
Splitting pattern: Since there are five d orbitals, the splitting occurs in a ratio of 2:3. In octahedral complexes, two orbitals are in the high energy level (collectively known as ‘eg’), and three orbitals are in the lower energy level (collectively known as t2g). In tetrahedral complexes, the opposite occurs; three orbitals are in the higher energy level and two in the lower energy level.
What is Strong Ligand?
A strong ligand or a strong field ligand is a ligand that can result in a higher crystal field splitting. This means, the binding of a strong field ligand causes a higher difference between the higher and lower energy level orbitals. Examples include CN– (cyanide ligands), NO2– (nitro ligand) and CO (carbonyl ligands).
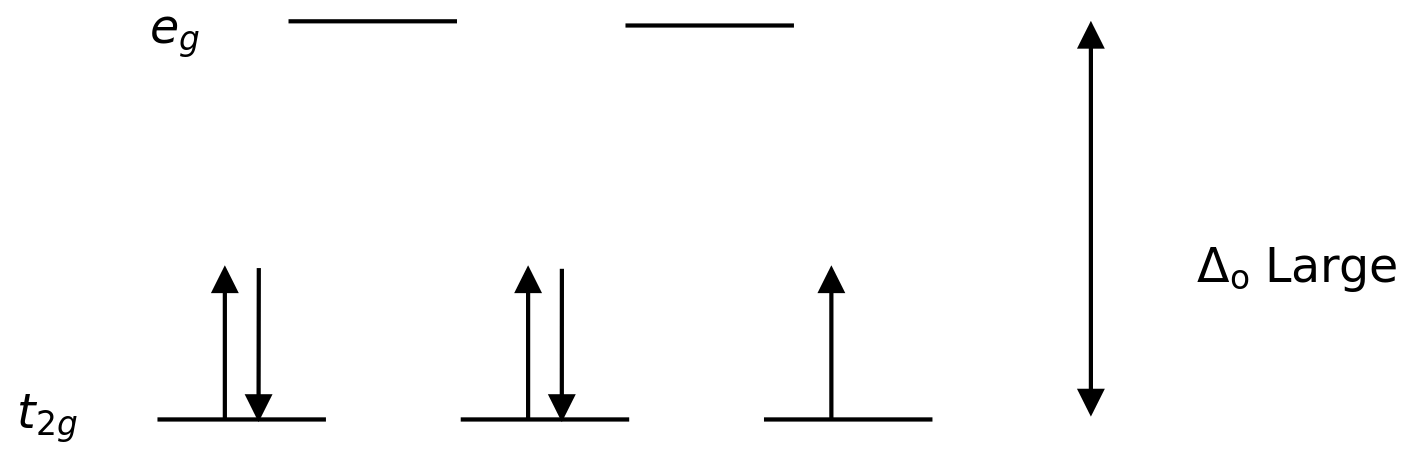
Figure 02: Low Spin Splitting
In the formation of complexes with these ligands, at first, the lower energy orbitals (t2g) are completely filled with electrons before filling to any other high energy level orbitals (eg). The complexes formed in this way are called “low spin complexes”.
What is Weak Ligand?
A weak ligand or a weak field ligand is a ligand that can result in a lower crystal field splitting. This means, the binding of a weak field ligand causes a lower difference between the higher and lower energy level orbitals.
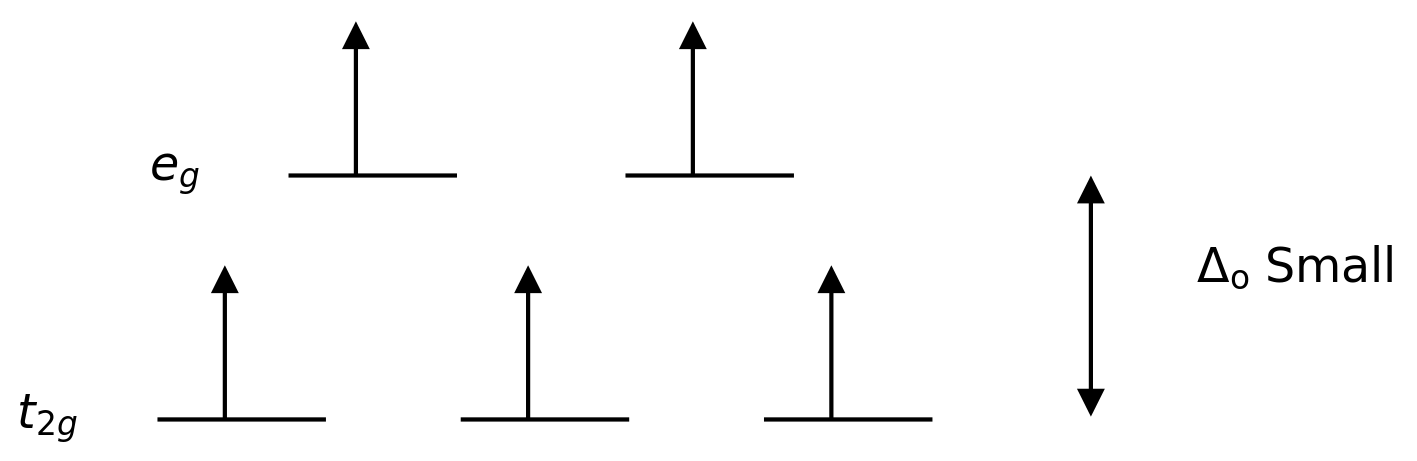
Figure 3: High Spin Splitting
In this case, since the low difference between the two orbital levels causes repulsions between electrons in those energy levels, the higher energy orbitals can be easily filled with electrons when compared to that in low energy orbitals. The complexes formed with these ligands are called “high spin complexes”. Examples of weak field ligands include I– (iodide ligand), Br– (bromide ligand), etc.
What is the Difference Between Strong Ligand and Weak Ligand?
Strong Ligand vs Weak Ligand | |
| A strong ligand or a strong field ligand is a ligand that can result in a higher crystal field splitting. | A weak ligand or a weak field ligand is a ligand that can result in a lower crystal field splitting. |
| Theory | |
| The splitting after binding a strong field ligand causes a higher difference between the higher and lower energy level orbitals. | The splitting of orbitals after binding a weak field ligand causes a lower difference between the higher and lower energy level orbitals. |
| Category | |
| The complexes formed with strong field ligands are called “low spin complexes”. | The complexes formed with weak field ligands are called “high spin complexes”. |
Summary – Strong Ligand vs Weak Ligand
Strong ligands and weak ligands are anions or molecules that cause splitting of d orbitals of a metal ion into two energy levels. The difference between strong ligands and weak ligands is that the splitting after binding a strong field ligand causes a higher difference between the higher and lower energy level orbitals whereas the splitting of orbitals after binding a weak field ligand causes a lower difference between the higher and lower energy level orbitals.
Reference:
1.Helmenstine, Anne Marie, D. “Ligand Definition.” ThoughtCo, Feb. 11, 2017. Available here
2.“Ligands.” Chemistry LibreTexts, Libretexts, 19 Jan. 2018. Available here
3.The Editors of Encyclopædia Britannica. “Ligand.” Encyclopædia Britannica, Encyclopædia Britannica, inc., 12 Aug. 2010. Available here
Image Courtesy:
1.’Octahedral crystal-field splitting’By English Wikipedia user YanA, (CC BY-SA 3.0) via Commons Wikimedia
2.’CFT-Low Spin Splitting Diagram-Vector’By Offnfopt, (Public Domain) via Commons Wikimedia
3.’CFT-High Spin Splitting Diagram-Vector’By Offnfopt, reference image created by YanA – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2F06ump59dobaorc2dZJqmlGLDtHnWnpikZZyetKK6w2g%3D