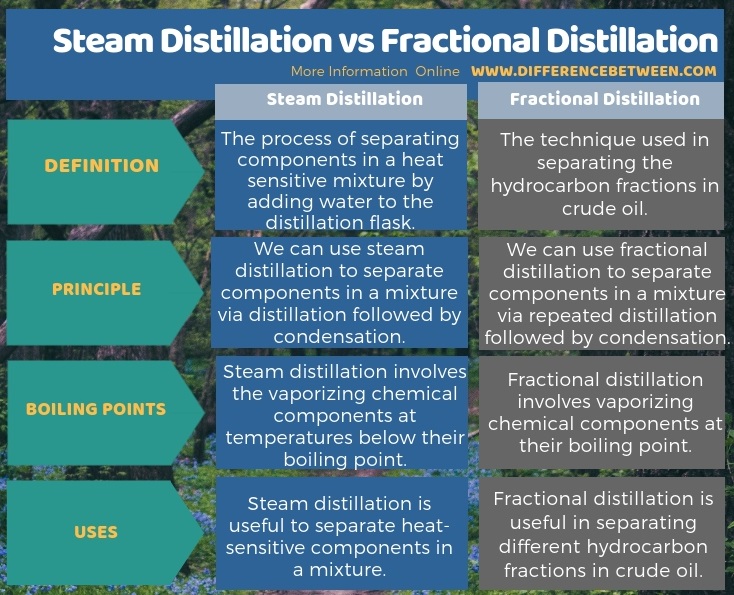
The key difference between steam distillation and fractional distillation is that the steam distillation separates heat sensitive components whereas the fractional distillation separates hydrocarbon fractions.
Distillation is the process of heating a liquid to create vapor that is collected when cooled separately from the original liquid. This process uses the differences in boiling points or volatility of different components in a mixture. There are several types of distillation techniques such as simple distillation, batch distillation, continuous distillation, steam distillation and fractional distillation.
CONTENTS
1. Overview and Key Difference
2. What is Steam Distillation
3. What is Fractional Distillation
4. Side by Side Comparison – Steam Distillation vs Fractional Distillation in Tabular Form
5. Summary
What is Steam Distillation?
Steam distillation is a process of separating components in a heat sensitive mixture by adding water to the distillation flask. Therefore, it is useful as a purification technique to remove impurities in a compound. However, the components of the mixture should be volatile in order to successfully carry this process.

Figure 01: Steam Distillation Apparatus
In this process, we separate the components in the mixture by vaporizing them at boiling points lower than their actual boiling point. We should follow this principle because otherwise, some components may decompose before reaching the boiling point. Then we cannot separate them accurately. We can add water to the distillation flask in which the mixture to be separated is placed. We add water in order to drop down the boiling points of the components. Then we can heat the mixture while agitating it. As a result of this step, the components tend to vaporize quickly. Then the vapor pressure of the distillation flask increases. When this vapor pressure exceeds the atmospheric pressure, the mixture starts to boil. Since the mixture boils at low pressure (lower than the atmospheric pressure), the boiling point of the components also drops down.
What is Fractional Distillation?
Fractional distillation is a technique useful in separating the hydrocarbon fractions in crude oil. In this process, we can separate different hydrocarbons according to the differences between their boiling points. We call this separation process as “fractionation”.

Figure 02: Fractional Distillation Apparatus
When considering the process, first we should heat the crude oil to very high temperatures and pressures. As a result, crude oil starts to vaporize. The vapor enters the fractional distillation column. There is a temperature gradient along the column (bottom has a high temperature, and the top is cold). Since the vapor moves upwards in the column, vapor cools down. At the point where the temperature of the column equals the boiling point of a hydrocarbon in a vapor, that hydrocarbon tends to condense. Since the distillation column has several plates at different distances, we can collect the condensed vapor as liquids from these plates.
What is the Difference Between Steam Distillation and Fractional Distillation?
Steam distillation is a process of separating components in a heat sensitive mixture by adding water to the distillation flask whereas the fractional distillation is a technique useful in separating the hydrocarbon fractions in crude oil. This is the key difference between steam distillation and fractional distillation. Furthermore, we use repeated distillations in fractional distillation technique, but we use only one distillation step in steam distillation. Moreover, another difference between steam distillation and fractional distillation is that in steam distillation, we can separate the components at low temperatures than the actual boiling points whereas in fractional distillation, the components in crude oil are separated at their boiling points.
The below infographic presents the difference between steam distillation and fractional distillation in tabular form for quick reference.
Summary – Steam Distillation vs Fractional Distillation
Distillation is a very useful technique that we can use to separate the components in a mixture using heat. Steam distillation and fractional distillation are two such techniques. The difference between steam distillation and fractional distillation is that steam distillation separates heat sensitive components whereas fractional distillation separates hydrocarbon fractions.
Reference:
1. Helmenstine, Anne Marie. “Learn What Distillation Means in Chemistry.” ThoughtCo, ThoughtCo. Available here
Image Courtesy:
1.”Steam dist”By Joanna Kośmider – Own work, (Public Domain) via Commons Wikimedia
2.”Fractional distillation lab apparatus”By John Kershaw and Theresa knott (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2F056YpmWUnsC1tculmK2hn6N6orrDZp2rmZOptrC6wKVknaGjqbatuMCtoKimXw%3D%3D