The key difference between spin-orbit coupling and Russell-Saunders effect is that spin-orbit coupling describes the interaction between a particle’s spin with its’ orbital motion whereas Russell-Saunders coupling effect describes the coupling of orbital angular momenta of several electrons.
The term coupling in analytical chemistry mainly refers to the interaction between chemical components such as orbitals and electrons. Spin-orbit coupling and Russel-Saunders effect are two such coupling forms. Generally, Russell-Saunders effect is named as LS coupling and refers to the interaction between angular momenta of L and S orbitals.
CONTENTS
1. Overview and Key Difference
2. What is Spin-orbit Coupling
3. What is Russell-Saunders Effect
4. Side by Side Comparison – Spin-orbit Coupling vs Russell-Saunders Effect in Tabular Form
5. Summary
What is Spin-Orbit Coupling?
Spin-orbit coupling is a type of interaction between the spin of a particle and its motion inside a potential. It is a type of relativistic interaction. A common example in chemistry for spin-orbit coupling is the spin-orbit interaction which leads to the shifts in the atomic energy levels of an electron due to the electromagnetic interaction between the magnetic dipole of an electron and its orbital motion, along with the electrostatic field of the positively charged atomic nucleus. We can detect spin-orbit coupling as a splitting of spectral lines. It appears as a Zeeman effect that is produced by two relativistic effects: the apparent magnetic field seen from the electron’s perspective and the magnetic moment of the electron.
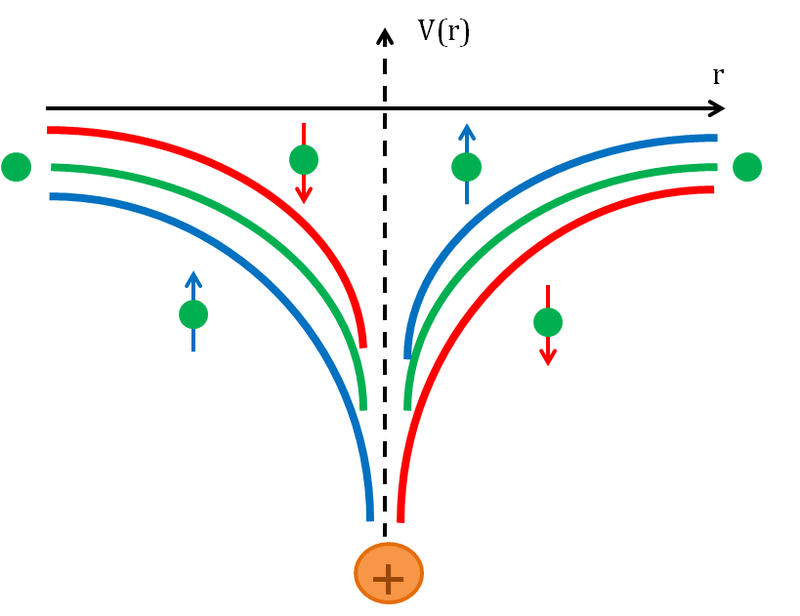
Figure 01: Spin-Orbit Coupling Potential
The phenomenon of spin-orbit coupling is important in the field of spintronics in order to conduct the electrons in semiconductors and other materials. Moreover, spin-orbit coupling is the cause for magnetocrystalline anisotropy and the spin-hall effect. We can observe spin-orbit coupling in atomic energy levels and in solids as well.
What is Russell-Saunders Effect?
Russell-Saunders effect is a type of coupling effect in analytical chemistry in which all the angular momenta of several electrons are strongly coupled together, forming the total electronic orbital angular momentum of the atom. This phenomenon is usually named LS coupling because L stands for orbital angular momentum and S stands for spin angular momentum. This is one of the simplest coupling schemes in chemistry.
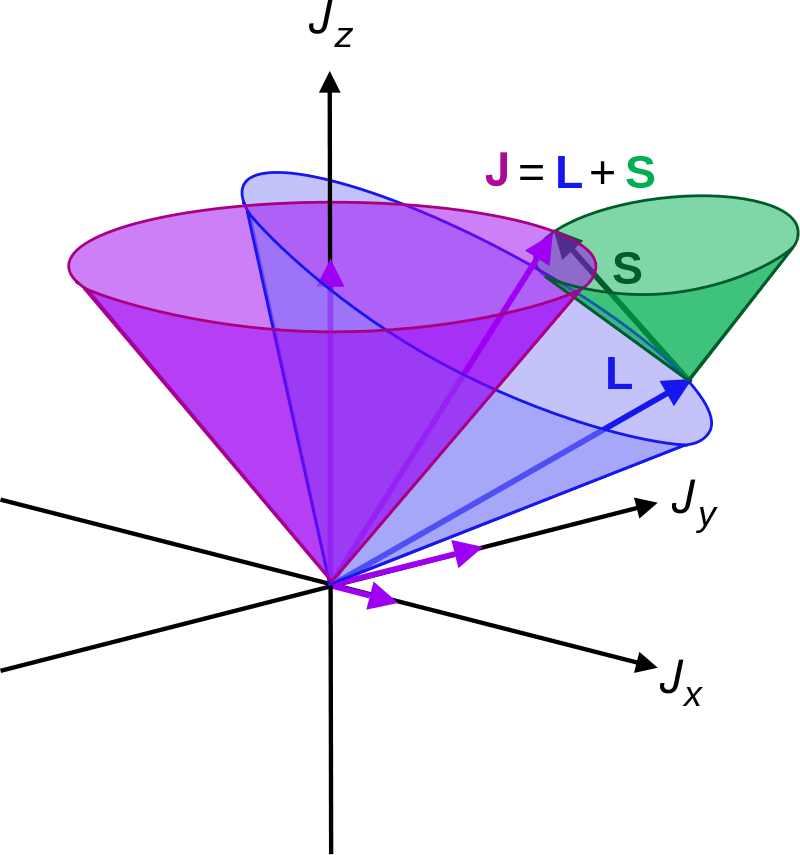
Figure 02: LS Coupling
Russell-Saunders coupling can be observed mainly in light atoms that usually have a value that is less than 30 for the atomic number. In these small atoms, electron spin (s) interacts with each other, forming a total spin angular momentum (S). The same process happens with electron orbitals (l) forming a total orbital angular momentum (L). The interaction between these L and S momenta is named LS coupling or Russell-Saunders effect. However, in large magnetic fields, we can observe these two momenta decoupling. Therefore, this phenomenon is suitable for systems with small and weak external magnetic fields.
What is the Difference Between Spin-orbit Coupling and Russell-Saunders Effect?
The term coupling in analytical chemistry mainly refers to the interaction between chemical components such as orbitals and electrons. The key difference between spin-orbit coupling and Russell-Saunders effect is that spin-orbit coupling describes the interaction between a particle’s spin with its’ orbital motion whereas Russell-Saunders coupling effect describes the coupling of orbital angular momenta of several electrons.
Below is a summary of the difference between spin-orbit coupling and Russell-Saunders effect in tabular form.
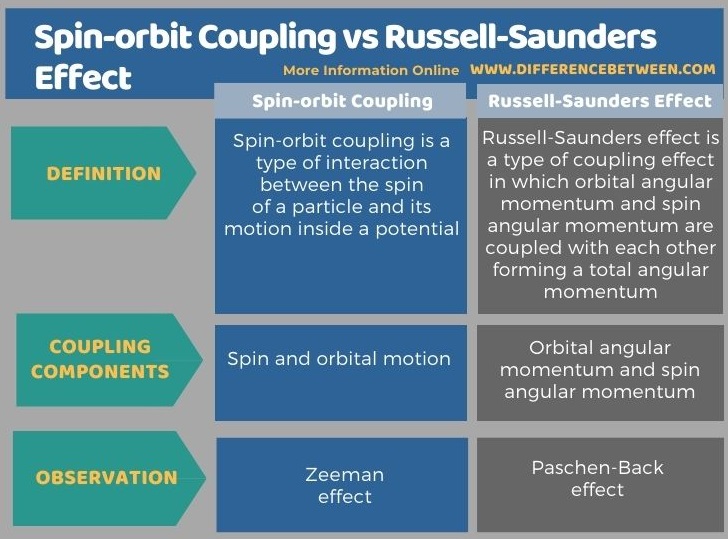
Summary – Spin-orbit Coupling vs Russell-Saunders Effect
The term coupling in analytical chemistry mainly refers to the interaction between chemical components such as orbitals and electrons. The key difference between spin orbit coupling and Russell-Saunders effect is that spin orbit coupling describes the interaction between a particle’s spin with its’ orbital motion whereas Russell-Saunders coupling effect describes the coupling of orbital angular momenta of several electrons.
Reference:
1. “Spin-Orbit Coupling.” Chemistry LibreTexts, Libretexts, 15 Aug. 2020, Available here.
Image Courtesy:
1. “Spin-orbit coupling potential” By Food95 – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “LS coupling” By Maschen – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2Fz6KlZqeil7a1ecKorKmkmaO0bq3NnWSrraOosq24jKyYrqaUmr%2B0ecSfnZ6bpGQ%3D