The key difference between species and phase in solution is that species of a solution refers to the chemical components present in the solution whereas phase in a solution refers to the visibly different forms of substances present in a solution.
A solution is a mixture of a solvent and a solute(s). The solutes are dissolved in the solvent. Some solutes dissolve in the solvent as they are while others dissolve through ionization. Therefore, the species present in the solution depends on the ionization ability of the compound in the solvent. There are two types of solutions as homogeneous solutions and heterogeneous solutions, depending on the phase of the matter.
CONTENTS
1. Overview and Key Difference
2. What are Species in Solution
3. What is a Phase in Solution
4. Side by Side Comparison – Species vs Phase in Solution in Tabular Form
5. Summary
What are Species in Solution?
Species in a solution refers to the chemical components that form from the dissolution of a solute in a solvent when forming the solution. Some species dissolve in the solvent as they are. For example, glucose dissolution forms an aqueous glucose solution, which contains glucose molecules that have not undergone any change. Here, the chemical species in the solution are the glucose molecules.
Sometimes, ionic compounds dissolve in the solvent through ionization. That means; the compound dissociates into its ionic components upon dissolution in the solvent. In this case, the chemical species present in the solution are the ionic components, not the molecule that was dissolved. Therefore, the species in the solution can vary depending on the ionization ability of the solute.
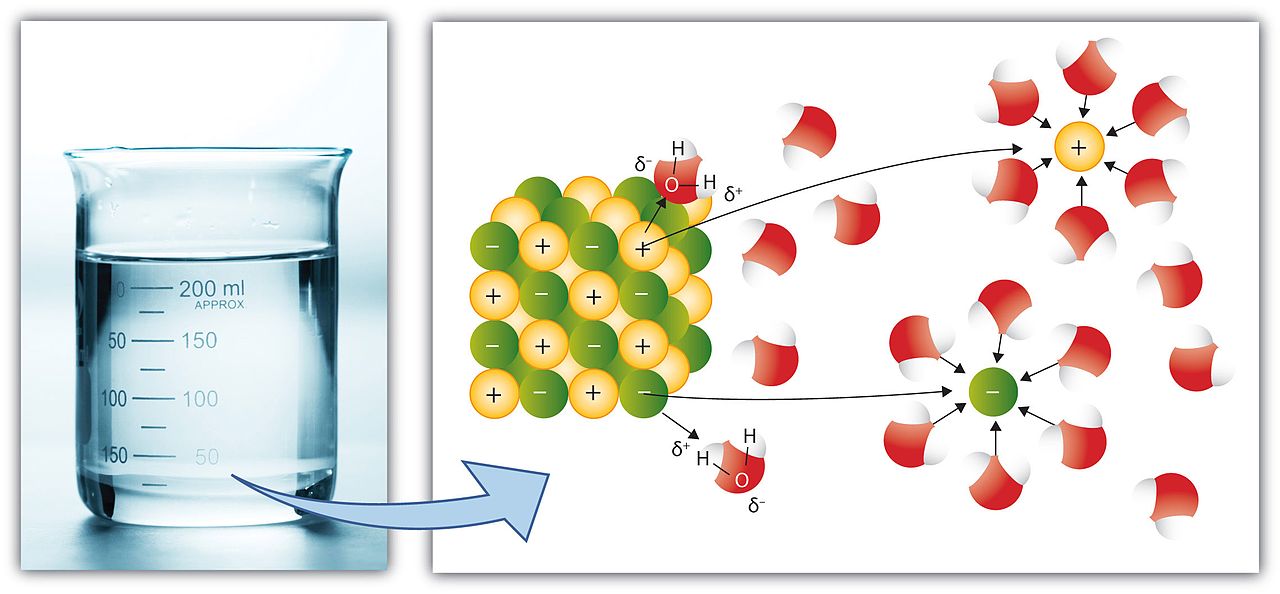
Figure 01: Ionic Species in Water
When explaining the properties of a solution, it is important to know the chemical species present in the solution. For instance, when we are expressing the concentration of a solution, we usually refer to the concentration of the dissolved solute or ions. Moreover, the boiling point of the solution, solubility of another component in a solution, and many other solution properties are dependent on the species present in the solution.
What is Phase in Solution?
Phase in a solution refers to the presence or absence of one or more phases of matter in the same solution. Here, we can categorize solutions into two types as homogeneous solutions and heterogeneous solutions.

Figure 02: Milk is a Heterogeneous Solution
A homogeneous solution is also called a single-phase solution because it has all its matter in the same phase. That means, the solutes and solvent are in the same phase, and we cannot observe different phases in these solutions. In contrast, heterogeneous solutions are multiple-phased solutions. That is; these solutions have two or more phases in the same solution. For example, emulsions have a liquid and solid phase in the same solution.
What is the Difference Between Species and Phase in Solution?
The key difference between species and phase in solution is that species of a solution refers to the chemical components present in the solution, whereas phase in a solution refers to the visibly different forms of substances present in a solution. Moreover, molecules or ions are the components of a species in a solution, whereas liquid and solid phases are the components of a species in a solution.
The following infographic summarizes the difference between species and phase in solution.
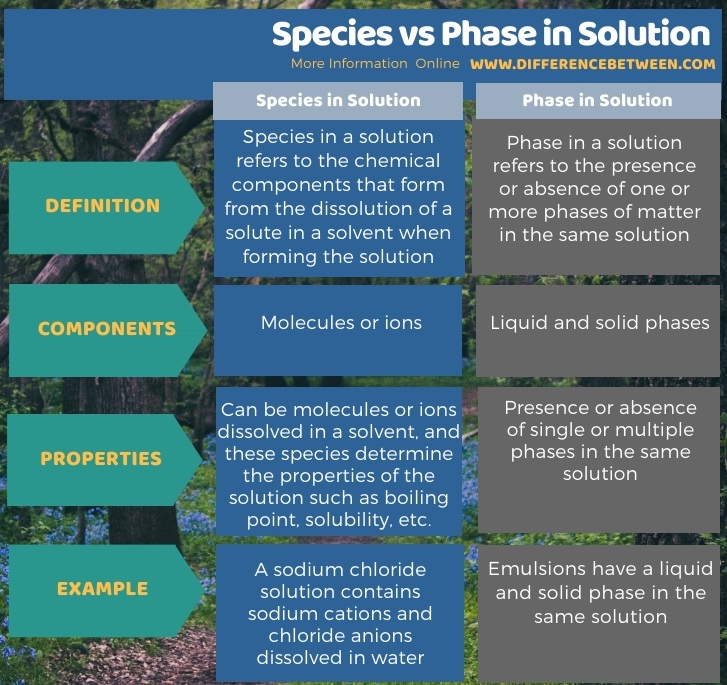
Summary – Species vs Phase in Solution
A solution is a mixture of a solvent and a solute(s). The solute(s) is dissolved in the solvent. The species in solution and phase in solution are important terms when expressing the properties of a solution. The key difference between species and phase in solution is that species of a solution refers to the chemical components present in the solution, whereas phase in a solution refers to the visibly different forms of substances present in a solution.
Reference:
1. Helmenstine, Anne Marie. “The Difference Between Heterogeneous and Homogeneous Mixtures.” ThoughtCo, Jan. 29, 2020, Available here.
2. “Thermodynamic Activity.” Wikipedia, Wikimedia Foundation, 4 Oct. 2019, Available here.
Image Courtesy:
1. “Sodium chloride dissolution” By Andy Schmitz – 2012 Book Archive (CC BY 3.0) via Commons Wikimedia
2. “cute little milk” By hobvias sudoneighm (CC BY 2.0) via Flickr
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2Fz56aop2jYq6vsIypn5qrlWK2r3nSqKOurJmku3A%3D