Key Difference – SN1 vs E1 Reactions
SN1 reactions are substitution reactions in which new substituents are substituted by replacing existing functional groups in organic compounds. E1 reactions are elimination reactions in which existing substituents are removed from the organic compound. The key difference between SN1 and E1 reactions is that SN1 reactions are substitution reactions whereas E1 reactions are elimination reactions.
SN1 and E1 reactions are very common in organic chemistry. These reactions result in the formation of new compounds via bond breakage and formations.
CONTENTS
1. Overview and Key Difference
2. What is SN1 Reactions
3. What is E1 Reactions
4. Similarities Between SN1 and E1 Reactions
5. Side by Side Comparison – SN1 vs E1 Reactions in Tabular Form
6. Summary
What are SN1 Reactions?
SN1 reactions are nucleophilic substitution reactions in organic compounds. These are two-step reactions. Hence, the rate determining step is the carbocation formation step. SN1 reactions are known as unimolecular substitutions because the rate-determining step involves one compound. The compound that undergoes SN1 reaction is known as the substrate. When there is a suitable nucleophile present, a leaving group is removed from the organic compound forming a carbocation intermediate compound. Then the nucleophile is attached to the compound in the second step. This gives a new product.
The first step of an SN1 reaction is the slowest reaction while the second step is faster than the first step. The rate of the SN1 reaction depends on one reactant since it is a unimolecular reaction. SN1 reactions are common in compounds with tertiary structures. Because, higher the distribution of atoms, greater the stability of the carbocation. The carbocation intermediate is attacked by the nucleophile. That is because nucleophiles are rich with electrons and are attracted to the positive charge of the carbocation.
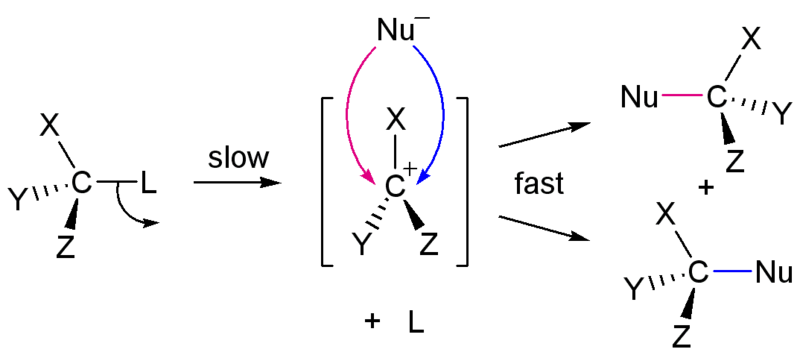
Figure 01: Mechanism of SN1 Reaction
Polar protic solvents such as water and alcohol can increase the reaction rate of SN1 reactions because these solvents can facilitate the formation of carbocation in the rate-determining step. A common example for an SN1 reaction is the hydrolysis of tert-butyl bromide in the presence of water. Here, water act as the nucleophile because the oxygen atom of the water molecule has lone electron pairs.
What are E1 Reactions?
E1 reactions are unimolecular elimination reactions. It is a two-step process, the first step being the rate-determining step because a carbocation intermediate is formed in the first step via leaving of a substituent. Presence of bulky groups in the starting compound facilitates the formation of carbocation. In the second step, another leaving group is removed from the compound.
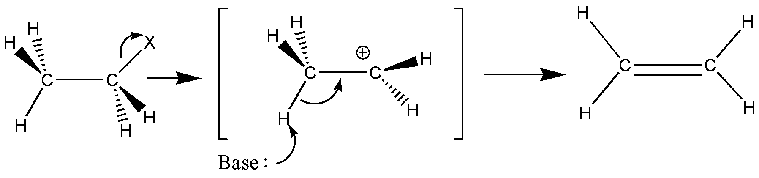
Figure 02: An E1 Reaction takes place I the Presence of a Weak Base
The E1 reaction has two major steps named as ionization step and deprotonation step. In the ionization step, the carbocation (positively charged) is formed whereas, in the deprotonation step, a hydrogen atom is removed from the compound as a proton. Eventually, a double bond is formed between two carbon atoms from which the leaving groups were eliminated. Hence, a saturated chemical bond becomes unsaturated after the completion of the E1 reaction. Two adjacent carbon atoms of the same compound are involved in E1 reactions.
Polar protic solvents facilitate E1 reactions because polar protic solvents are favourable for the formation of carbocation. Typically, E1 reactions can be observed regarding tertiary alkyl halides having bulky substituents. E1 reactions occur in either the complete absence of bases or in the presence of weak bases.
What are the Similarities Between SN1 and E1 Reactions?
- Bot SN1 and E1 Reactions include the formation of a carbocation.
- Polar protic solvents facilitate both types of reactions.
- Both reactions are unimolecular reactions.
- Both reactions are two-step reactions.
- Both reactions have a rate-determining step.
- Better the leaving group, higher the reaction rate of both SN1 and E1 Reactions.
- Both SN1 and E1 Reactions can be found typically regarding compounds having tertiary structures.
- Rearrangements can take place in the carbocation of both reactions.
What is the Difference Between SN1 and E1 Reactions?
SN1 vs E1 Reactions | |
| SN1 reactions are nucleophilic substitution reactions in organic compounds. | E1 reactions are unimolecular elimination reactions. |
| Requirement of a Nucleophile | |
| SN1 reactions require a nucleophile in order to form the carbocation. | E1 reactions do not require a nucleophile to form the carbocation. |
| Process | |
| SN1 reactions include substitution of a nucleophile. | E1 reactions include the elimination of a functional group. |
| Double Bond Formation | |
| No double bond formations can be observed in SN1 reactions. | A double bond is formed between two carbon atoms in E reactions. |
| Unsaturation | |
| There is no unsaturation take place after the completion of SN1 reactions. | A saturate chemical becomes unsaturated after the completion of an E1 reaction. |
| Carbon Atoms | |
| One central carbon atom is involved in SN1 reactions. | Two adjacent carbon atoms of the same compound are involved in E1 reactions. |
Summary – SN1 vs E1 Reactions
SN1 reactions are nucleophilic substitution reactions. E1 reactions are elimination reactions. Both types of reactions are unimolecular reactions because the rate-determining step of these reactions involves a single molecule. Although these two types of reaction share many similarities, there are some differences as well. The difference between SN1 and E1 reactions is that SN1 reactions are substitution reactions whereas E1 reactions are elimination reactions.
Reference:
1.“SN1 Reaction.” Wikipedia, Wikimedia Foundation, 21 Mar. 2018. Available here
2.“E1 Reactions.” Chemistry LibreTexts, Libretexts, 21 July 2016. Available here
Image Courtesy:
1.’SN1 reaction mechanism’By Calvero (Public Domain) via Commons Wikimedia
2.’E1EliminationReaction’By Pdavis68 (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2FzWpkmqaUYsO0ecRqZKudkZjBqrvNrGY%3D