The key difference between Schiff base and Schiff’s reagent is that the term Schiff base refers to either secondary ketimines or secondary aldimines, whereas the term Schiff’s reagent refers to a reagent used to test for aldehydes and ketones.
Schiff base and Schiff’s reagent were named after the scientist Hugo Schiff. These terms are used in Schiff test, which detects aldehydes and ketones in a given sample. These two terms are used to name a group of particular organic compounds.
CONTENTS
1. Overview and Key Difference
2. What is Schiff Base
3. What is Schiff’s Reagent
4. Side by Side Comparison – Schiff Base vs Schiff’s Reagent in Tabular Form
5. Summary
What is Schiff Base?
Schiff base is an organic compound having the chemical formula R2C=NR’. Here, R groups are not equal to hydrogen atoms; they are either alkyl or aryl groups. The compounds that fall under the category of Schiff base belong to a sub-class of imines. These can be either secondary ketimines or secondary aldimines. Usually, we use the term Schiff base for the chemical species that can act as ligands when forming coordination complexes with metal ions. Although some complexes exist as natural components, e.g. corrin, most Schiff bases are artificial.
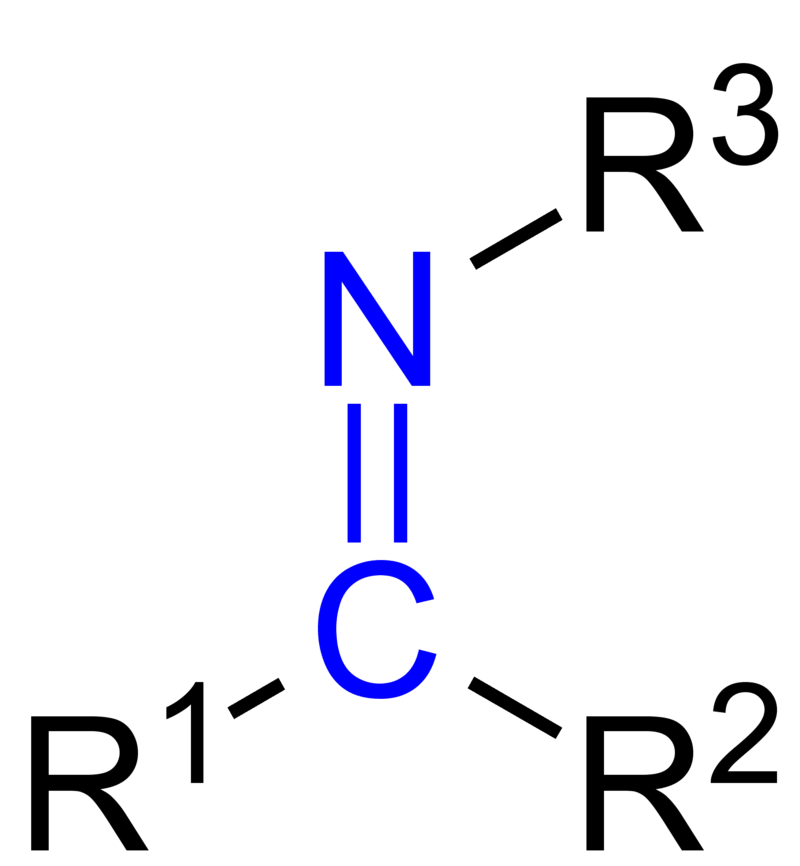
Figure 01: General Structure of a Schiff Base
When considering the synthesis of a Schiff base, we can produce it via nucleophilic addition of either an aliphatic or aromatic amine and a carbonyl compound such as an aldehyde or a ketone. This reaction produces a hemiaminal we can perform dehydration with, to generate an imine.
What is Schiff’s Reagent?
Schiff’s reagent is a chemical reagent which is used test for aldehydes and ketones. It is a liquid containing fuchsin dye. The dye in this solution is decolourized with sulfur dioxide. When we use this reagent in Schiff test, we can observe that the pink colour of the reagent is restored if the sample contains aldehydes and if it has ketones but no aldehydes, the pink colour cannot be observed. However, aliphatic ketones and aromatic aldehydes tend to restore the pink colour slowly.
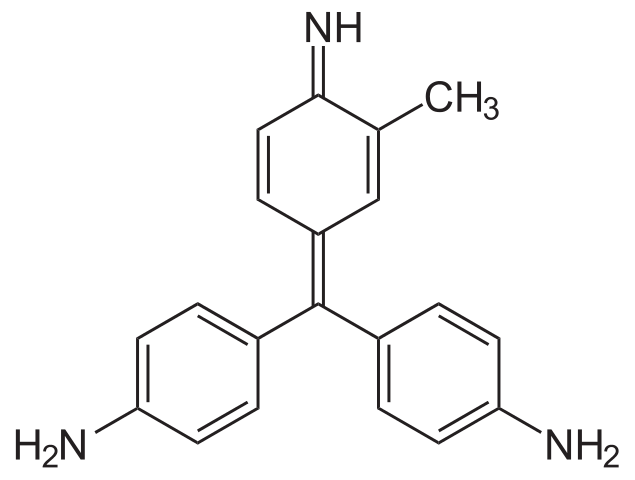
Figure 02: The Structure of the Fuchsin Dye
Usually, Schiff’s reagents are coloured due to the absorbance of visible wavelengths by its central quinoid structure. upon sulfonation, the reagent gets decolourized. Here, the central carbon atom of the dye undergoes sulfonation by sulfurous acid or its conjugate base. The further reaction between aldehydes and Schiff’s reagent forms multiple reaction products.
What is the Difference Between Schiff Base and Schiff’s Reagent?
The terms Schiff base and Schiff’s reagent are used in analytical chemistry. The key difference between Schiff base and Schiff’s reagent is that the term Schiff base refers to either secondary ketimines or secondary aldimines, whereas the term Schiff’s reagent refers to a reagent used to test for aldehydes and ketones. Moreover, the Schiff base is a particular organic molecule, whereas Schiff’s reagent is a solution containing fuchsin dye.
Below infographic tabulates the differences between Schiff base and Schiff’s reagent.
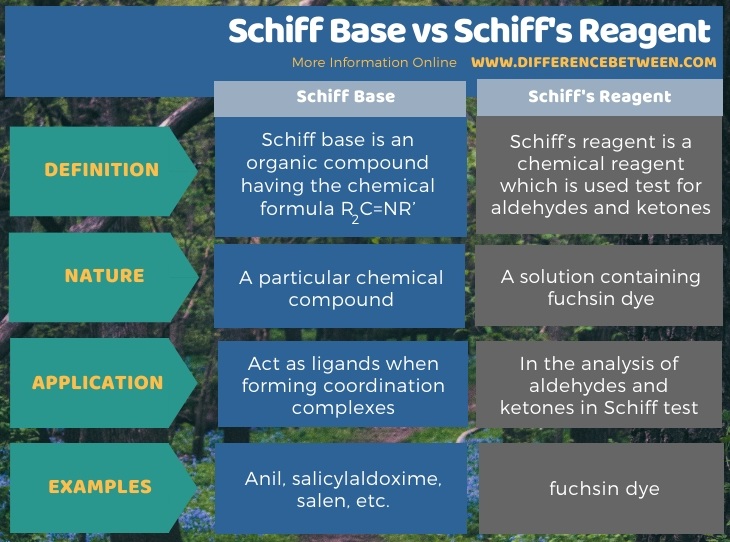
Summary – Schiff Base vs Schiff’s Reagent
The terms Schiff base and Schiff’s reagent are used in analytical chemistry. The key difference between Schiff base and Schiff’s reagent is that the term Schiff base refers to either secondary ketimines or secondary aldimines, whereas the term Schiff’s reagent refers to a reagent used to test for aldehydes and ketones. Moreover, the Schiff base is a particular organic molecule, whereas the Schiff’s reagent is a solution containing fuchsin dye.
Reference:
1. “Schiff’s Reagent.” A Dictionary of Biology, Encyclopedia.com, 14 Feb. 2020, Available here.
2. “Schiff Base.” Wikipedia, Wikimedia Foundation, 21 Dec. 2019, Available here.
3. “Schiff Test.” Wikipedia, Wikimedia Foundation, 14 Dec. 2019, Available here.
Image Courtesy:
1. “Imino Group V.1” By Jü – Own work (Public Domain) via Commons Wikimedia
2. “Fuchsin” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2FwqGgn55dl660sYyapZ1lo5i1qrLFrGSrnZGcsq%2FAjg%3D%3D