The key difference between primary and secondary allylic carbocations is that primary allylic carbocation is less stable than secondary allylic carbocation.
An allylic carbocation is a resonance stabilized carbon structure. It is an ion containing a positive charge. In these ions, the positive ion is placed on the allylic carbon atom (an allylic carbon atom is the adjacent atom to a double bond). Primary allylic carbocation is an allylic carbocation where the positive charge is placed on a primary carbon atom while secondary allylic carbocation is an allylic carbocation where the positive charge is placed on a secondary carbon atom.
CONTENTS
1. Overview and Key Difference
2. What are Primary Allylic Carbocations
3. What are Secondary Allylic Carbocations
4. Side by Side Comparison – Primary vs Secondary Allylic Carbocations in Tabular Form
5. Summary
What are Primary Allylic Carbocations?
Primary allylic carbocation is an allylic carbocation where the positive charge is placed on a primary carbon atom. It is named as a carbocation because it contains a positive charge on a carbon atom. Usually, an allylic carbocation carries a +1 positive charge. A primary carbon atom is a carbon atom that is attached to two hydrogen atoms and a double bond. Typically, a neutral carbon atom forms four covalent bonds, and one covalent bond is removed when it forms a cation. A primary carbon atom contains only one aryl or alkyl group attached to it while other bonds are C-H bonds.
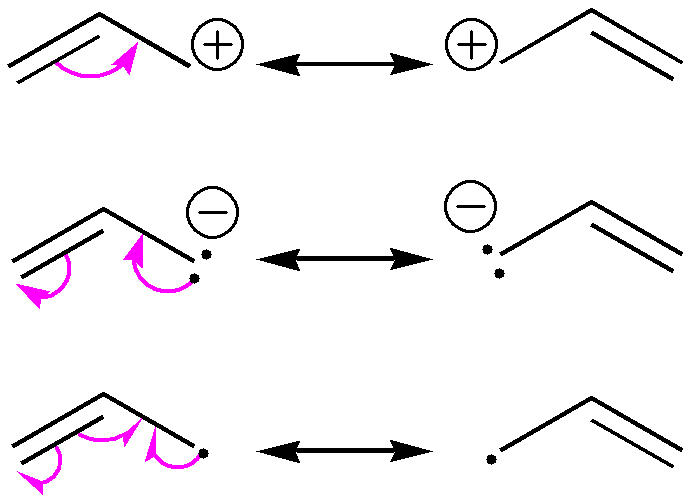
Figure 01: Allylic Resonance
Generally, a molecule containing double bonds between carbon atoms can have resonance stabilized structures. Resonance means that electrons in the pi bond of the double bond are distributed throughout the molecule as a delocalized system where the stability is increased than in a normal molecule. Therefore, if we are going to name a compound as a primary allylic carbocation, that particular compound should have a positive charge on allylic carbon atoms in all possible resonance structure of that molecule.
What are Secondary Allylic Carbocations?
Secondary allylic carbocation is an allylic carbocation where the positive charge is placed on a secondary carbon atom. It is named as a carbocation because it contains a positive charge on a carbon atom. Usually, an allylic carbocation carries a +1 positive charge. A secondary carbon atom is a carbon atom that is attached to one hydrogen atom, a double bond and an alkyl or aryl group. Typically, a neutral carbon atom forms four covalent bonds where one covalent bond is removed when it forms a cation. A secondary carbon atom contains two aryl or alkyl group attached to it while the other bond is a C-H bonds.
Sometimes, the term secondary allylic carbocation is used when only one resonance structure of a particular compound contains the positive charge on a secondary carbon atom. More importantly, secondary allylic carbocations are more stable than primary allylic carbocations because they have two aryl or alkyl groups attached to the carbon atom bearing the positive charge (aryl or alkyl groups are electron-withdrawing groups so, they can minimize the positive charge on the carbon atom).
What is the Difference Between Primary and Secondary Allylic Carbocations?
Allylic carbocations are chemical structures where the positive charge is on the allylic carbon atom of the molecule. The allylic carbon atom is the carbon atom that is adjacent to a double bond. Primary allylic carbocation is an allylic carbocation where the positive charge is placed on a primary carbon atom while secondary allylic carbocation is an allylic carbocation where the positive charge is placed on a secondary carbon atom. The key difference between primary and secondary allylic carbocations is that the primary allylic carbocation is less stable than the secondary allylic carbocation.
Below tabulation summarizes the differences between primary and secondary allylic carbocations.
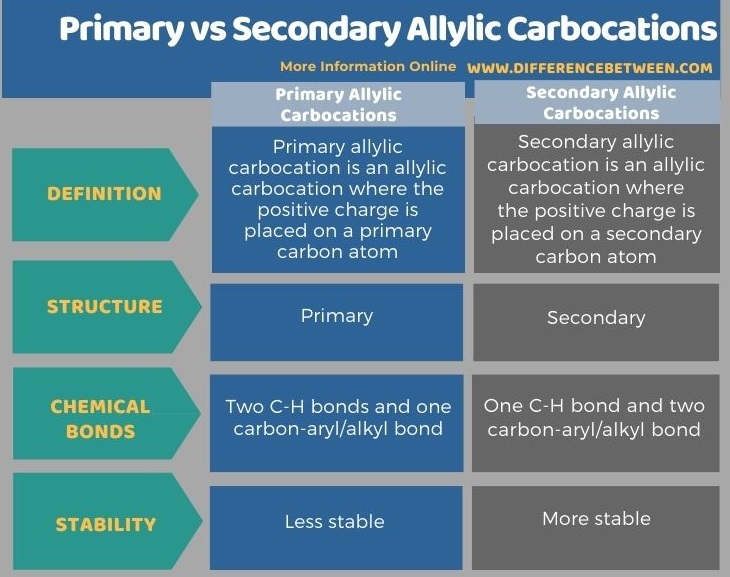
Summary – Primary vs Secondary Allylic Carbocations
Allylic carbocations are chemical structures where the positive charge is on the allylic carbon atom of the molecule. The allylic carbon atom is the carbon atom that is adjacent to a double bond. The key difference between primary and secondary allylic carbocations is that the primary allylic carbocation is less stable than the secondary allylic carbocation.
Reference:
1. “Secondary Allylic Carbocation.” Chemistry LibreTexts, Libretexts, 14 July 2020, Available here.
Image Courtesy:
1. “Allylic resonance” By User Tor Svensson – Structure drawn by me (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2680aKkmqqpYq6vsIysnJynnpmus8WMmqOlsZyesG6vwKuZqJuRqbawutJo