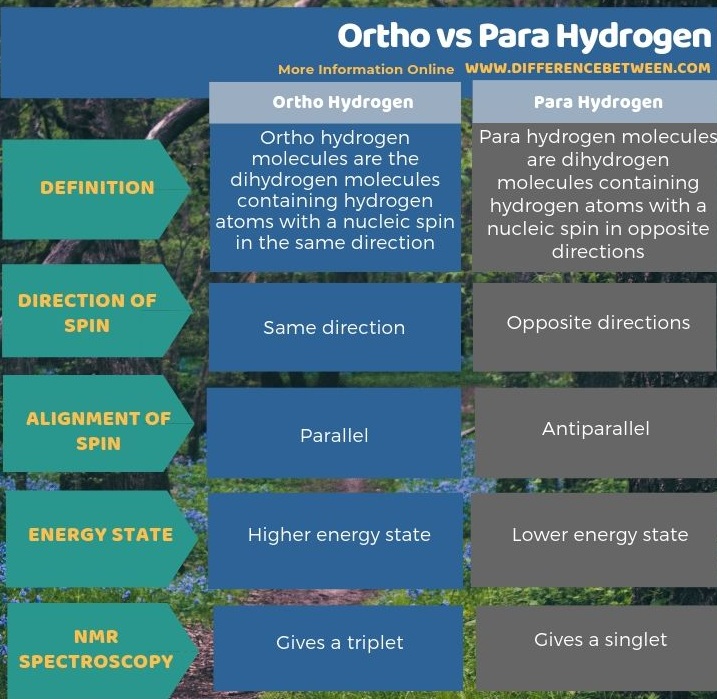
The key difference between ortho and para hydrogen is that ortho hydrogen molecules have spins of two nuclei in the same direction whereas para hydrogen molecules have spins of two nuclei in opposite directions.
Molecular hydrogen in a hydrogen molecule (H2) can be found in two forms as ortho hydrogen and para hydrogen. We categorize them as such depending on the alignment of the nuclear spins of these atoms. Therefore, we often refer to them as spin isomers.
CONTENTS
1. Overview and Key Difference
2. What is Ortho Hydrogen
3. What is Para Hydrogen
4. Side by Side Comparison – Ortho vs Para Hydrogen in Tabular Form
5. Summary
What is Ortho Hydrogen?
Ortho hydrogen molecules are the dihydrogen molecules containing hydrogen atoms with a nucleic spin in the same direction. In other words, the spin of two atoms is aligned parallel to each other. It is a spin isomer of para hydrogen.
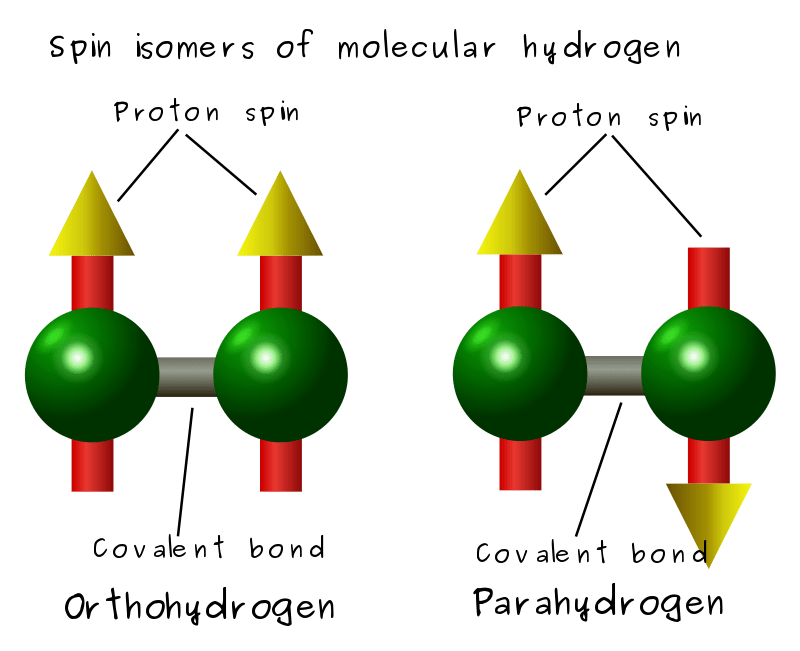
Figure 01: Ortho and Para Hydrogen Comparison
However, this isomer occurs in a higher energy state than the para hydrogen isomer. Furthermore, in NMR spectroscopy, ortho hydrogen forms a triplet state.
What is Para Hydrogen?
Para hydrogen molecules are dihydrogen molecules containing hydrogen atoms with a nucleic spin in opposite directions. This means the nuclear spin of each atom in the H2 molecule is opposite to each other. Furthermore, it is a spin isomer of ortho hydrogen. The spin atoms of two hydrogen atoms are also aligned antiparallel. Moreover, this isomer occurs in a lower energy state than the ortho isomer. Furthermore, in NMR spectroscopy, this hydrogen gives a singlet state.
What is the Difference Between Ortho and Para Hydrogen?
The key difference between ortho and para hydrogen is that ortho hydrogen molecules have spins of two nuclei in the same direction whereas para hydrogen molecules have spins of two nuclei in opposite directions. When considering the energy of these molecules, ortho hydrogen has a higher energy state than para hydrogen. Moreover, in NMR spectroscopy, ortho hydrogen gives triplet state while para hydrogen gives singlet state.
Summary – Ortho vs Para Hydrogen
Basically, ortho and para hydrogen are two types of H2 molecules we can categorize according to the spin of the hydrogen atoms. The key difference between ortho and para hydrogen is that ortho hydrogen molecules have spins of two nuclei in the same direction whereas para hydrogen molecules have spins of two nuclei in opposite directions.
Reference:
1.“Ortho and Para Hydrogen.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
Image Courtesy:
1. “Spinisomers of molecular hydrogen” By GKFXtalk (vectorized), en:User:Xaa (Jim Farris) (original) – Vectorized by myself (GKFXtalk) from also File:Spinisomers_of_molecular_hydrogen.png (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2670a2fqGWRo7FuvMCrmGagqZm%2FsLPEp2Y%3D