The key difference between organic and inorganic phosphate is that the organic phosphates are phosphates of esters whereas the inorganic phosphates are salts of phosphoric acid.
Phosphates are chemical compounds consisting of phosphate anions (PO4– anion). The two main types of these compounds are organic phosphates and inorganic phosphates. These compounds have different chemical and physical properties; thus, different applications in the industry. Let us go into more details about them.
CONTENTS
1. Overview and Key Difference
2. What is Organic Phosphate
3. What is Inorganic Phosphate
4. Side by Side Comparison – Organic vs Inorganic Phosphate in Tabular Form
5. Summary
What is Organic Phosphate?
Organic phosphates are phosphates of esters. We call them “organophosphates”. These are esters of phosphoric acid. Since the chemical formula of phosphoric acid is H3PO4, an ester forms when this acid replaces a hydrogen atom of a hydrocarbon. As a result, the inorganic acid becomes organic. These organic phosphates are very useful for agricultural purposes. For example, we use organophosphate pesticides such as parathion, malathion, dichlorvos, etc. to control pests.
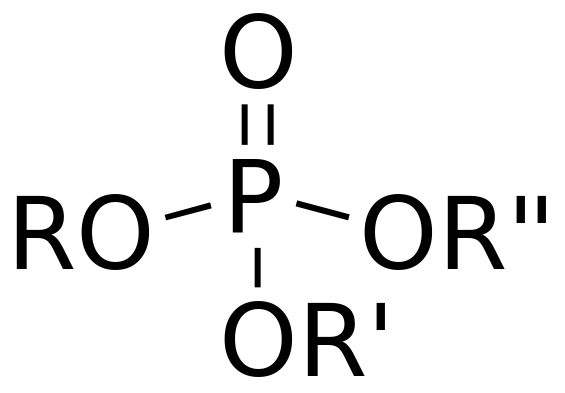
Figure 01: General Structure of Organophosphates
The organic groups of these compounds can link with each other to form new phosphate compounds. If these compounds contain hydroxyl groups, they have an acidic nature. This is because, in an aqueous solution, these compounds can release the proton in the hydroxyl group making the solution acidic. Then this ionized phosphate compound will attach with other organic groups forming new compounds. In addition to the use as fertilizers, these compounds are useful as additives, solvents, plasticizers, etc.
What is Inorganic Phosphate?
Inorganic phosphates are salts of phosphoric acid. In these compounds, we can see a phosphate group attached to a metal cation. Therefore, phosphate group acts as an anion. The overall charge of this anion is -3. This indicates that this anion can participate in the formation of monobasic, dibasic and tribasic salts. The phosphate group has a tetrahedral arrangement. Inorganic phosphates occur naturally as the salts of group 1 elements. ex: sodium (Na), potassium (K), calcium (Ca), etc.
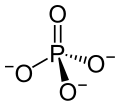
Figure 02: Phosphate Anion
The two major inorganic phosphate compounds are orthophosphates and condensed phosphates. Among them, orthophosphates are very reactive, and these are the simplest inorganic phosphates. They contain only one phosphate unit per molecule. Condensed phosphates contain more than one phosphate unit. These compounds are also useful as fertilizers, ex: Superphosphate and Triple superphosphate.
What is the Difference Between Organic and Inorganic Phosphate?
Organic phosphates are phosphates of esters. In organic phosphates, the phosphate groups and organic groups connect with each other via covalent bonds. Moreover, they have only organic groups attached to the phosphate group. Inorganic phosphates are salts of phosphoric acid. In inorganic phosphates, the phosphate anions and metal cations have an electrostatic attraction force between them. In addition, they have inorganic groups attached to the phosphate group other than the metal cation. These are the main difference between organic and inorganic phosphate.
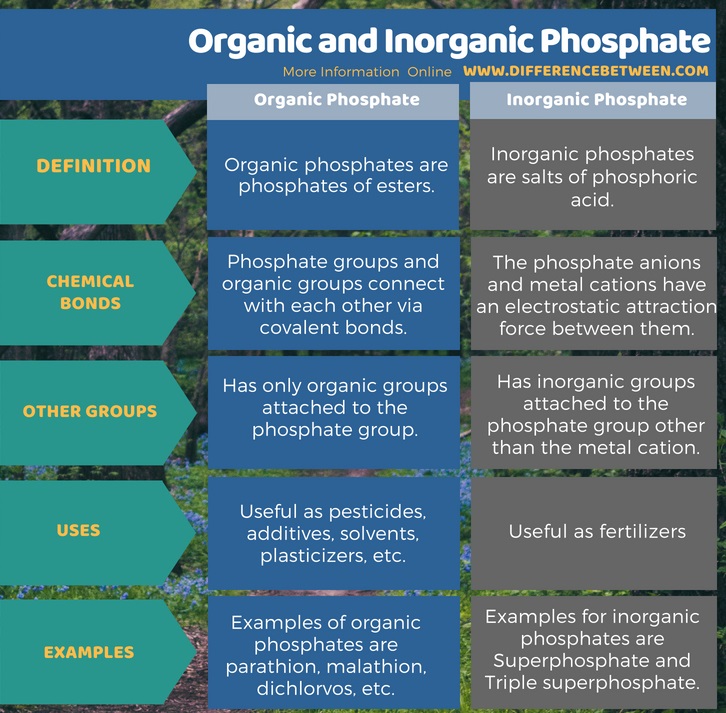
Summary – Organic vs Inorganic Phosphate
Phosphate compounds are in two types as organic phosphates and inorganic phosphates according to the chemical structure. The difference between organic and inorganic phosphate is that the organic phosphates are phosphates of esters whereas the inorganic phosphates are salts of phosphoric acid.
Reference:
1. “Organophosphate.” Wikipedia, Wikimedia Foundation, 3 July 2018. Available here
2. “Phosphate.” Wikipedia, Wikimedia Foundation, 4 July 2018. Available here
Image Courtesy:
1.’Organophosphate’By Calvero. – Selfmade with ChemDraw, (Public Domain) via Commons Wikimedia
2.’Phosphat-Ion’By NEUROtiker ⇌ – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2670aCYp6GTYq6vsIyipaiql5a7qq%2BMqZ%2Boq6CdrrWxjg%3D%3D