The key difference between monosubstituted and disubstituted alkene is that a monosubstituted alkene compound has a covalent bond with only one carbon, excluding the doubly bonded carbon atoms of the alkene, whereas disubstituted alkene compound has two carbon atoms bonded to the double-bonded carbon atoms of the alkene.
Alkenes are organic compounds having a double bond between two carbon atoms. The number of carbon atoms in the alkene and the number of double bonds can vary to a wide range. Moreover, the reactivity of an alkene depends on this chemical structure. In addition to these, substituents attached to the alkene can change the reactivity to a great degree.
Besides, the prefix “mono-“ means there is “only one” and the prefix “di-“ means there are two of the chemical moiety. Thus, this gives us an idea about the terms monosubstituted and disubstituted.
CONTENTS
1. Overview and Key Difference
2. What is Monosubstituted Alkene
3. What is Disubstituted Alkene
4. Side by Side Comparison – Monosubstituted vs Disubstituted Alkene in Tabular Form
5. Summary
What is Monosubstituted Alkene?
Monosubstituted alkenes are organic compounds having only one carbon atom bonded to one of the double-bonded carbon atoms in the alkene functional group. The substituted carbon atom belongs to either an aliphatic group or an aromatic group. For example, a monosubstituted alkene may have substituents such as methyl group, ethyl group, phenyl group, etc. Therefore, this type of organic compound contains 3 hydrogen atoms attached to the other positions.
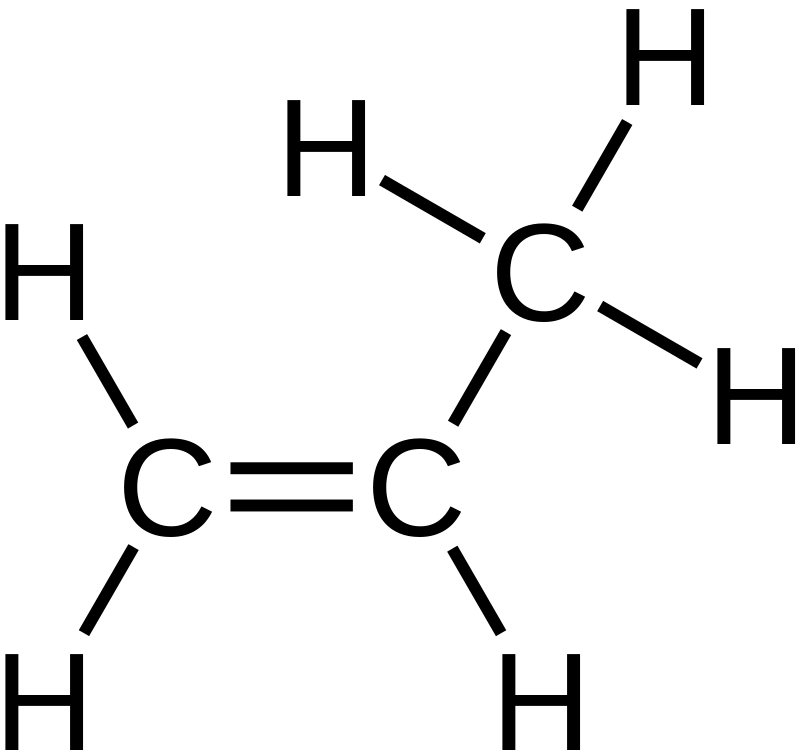
Figure 01: Propene is the Simplest Monosubstituted Alkene.
What is Disubstituted Alkene?
Disubstituted alkenes are organic compounds having two carbon atoms bonded to either the same carbon or two carbon atoms of the alkene functional group. Therefore, the double-bonded carbon atoms should be bonded to a total of two extra carbon atoms. Furthermore, some disubstituted alkenes contain two substituted carbon atoms belonging to the same substituent. Therefore, this type of organic compound contains 2 hydrogen atoms attached to the other positions.
What is the Difference Between Monosubstituted and Disubstituted Alkene?
The prefix “mono-“ means there is “only one” and the prefix “di-“ means there are two of the chemical moiety that we are considering. Thus, the key difference between monosubstituted and disubstituted alkene is that a monosubstituted alkene compound has a covalent bond with only one carbon, excluding the doubly bonded carbon atoms of the alkene, whereas disubstituted alkene compound has two carbon atoms bonded to the double-bonded carbon atoms of the alkene. Moreover, monosubstituted alkene has three hydrogen atoms while disubstituted alkene has two hydrogen atoms.
The following infographic summarizes the difference between monosubstituted and disubstituted alkene in tabular form.
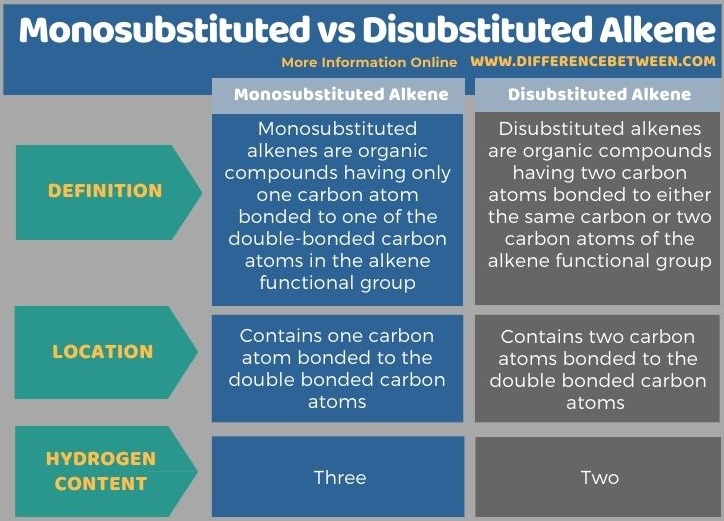
Summary – Monosubstituted vs Disubstituted Alkene
An alkene is an organic compound containing a double bond between two carbon atoms as the major functional group of the organic molecule. The key difference between monosubstituted and disubstituted alkene is that a monosubstituted alkene compound has a covalent bond with only one carbon, excluding the doubly bonded carbon atoms of the alkene, whereas disubstituted alkene compound has two carbon atoms bonded to the double-bonded carbon atoms of the alkene.
Reference:
1. “Disubstituted Alkene.” Chemistry LibreTexts, Libretexts, 24 Aug. 2020, Available here.
2. “Monosubstituted Alkene.” Chemistry LibreTexts, Libretexts, 24 Aug. 2020, Available here.
Image Courtesy:
1. “Propene-2D-flat” By Nothingserious – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265zqemrK2SqMGqwNStnJ1lkaOxbrDIrKybq6SewbbAxJ1kmqSbmrumew%3D%3D