The key difference between molten and aqueous electrolysis is that molten electrolysis produces elements of the analyte, whereas aqueous electrolysis produces an aqueous salt solution and a mixture of gases as the final product.
Molten and aqueous electrolysis are two types of electrolysis methods in analytical chemistry which are different from each other according to the properties of the electrolytic medium. The term “molten” refers to the liquid state of the analyte in the absence of water while the term “aqueous” refers to the liquid state in the presence of water.
CONTENTS
1. Overview and Key Difference
2. What is Molten Electrolysis
3. What is Aqueous Electrolysis
4. Side by Side Comparison – Molten vs Aqueous Electrolysis in Tabular Form
5. Summary
What is Molten Electrolysis?
Molten electrolysis is a technique in analytical chemistry that uses an electric current to separate the chemical elements in an analyte substance in its molten state. Generally, ionic compounds are used in this type of electrolysis method. This technique provides information on how we can extract metals such as aluminium and sodium from their molten ionic compounds using an electric current.
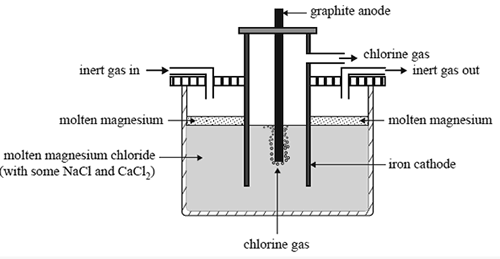
Figure 01: Extraction of Magnesium Metal
For example, aluminium is the most abundant metal on the earth’s surface, but it does not occur in a pure state in nature. Instead, it occurs as ionic compounds in minerals. Therefore, we have to separate aluminium from its compounds via electrolysis. Here, we use a molten ionic compound. An ionic compound forms due to the formation of a strong ionic bond that exists between cations and anions. In the solid-state of an ionic compound, the anions and cations are locked in a rigid structure so they cannot conduct electricity. Therefore, we cannot use a solid compound for electrolysis. But in its molten state, the ionic compound separates into anions and cations, allowing the molten state of the analyte to conduct electricity. We can get the molten state via melting the solid. Therefore, we can name the molten state of the analyte as an electrolyte.
During the process of molten electrolysis, the cations move towards the negative electrode while anions move towards the positive electrode. At the negative electrode (cathode), cations gain electrons and become atoms. At the positive electrode or the anode, ions lose electrons to become atoms.
What is Aqueous Electrolysis?
Aqueous electrolysis is a technique in analytical chemistry that uses an electric current to separate the chemical elements in an analyte substance in its aqueous state. This type of electrolysis is important in obtaining particular substances or gases. For example, if we pass an electric current through water, it forms hydrogen gas and oxygen gas.
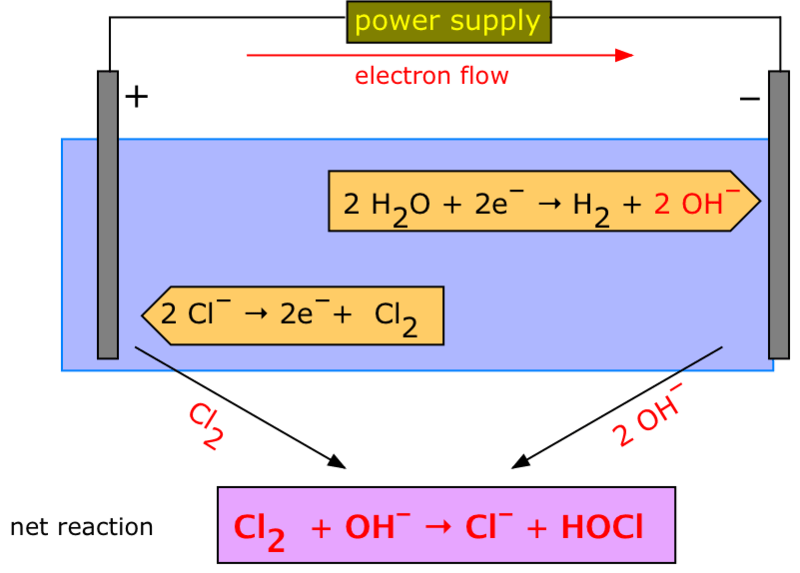
Figure 02: Electrolysis of Water
In the process of aqueous electrolysis, an electric current is passed through an ionized electrolyte; here the cations move towards the anode and anions move towards the cathode. This type of system is called an electrolytic cell. There are many applications of aqueous electrolysis, including electroplating, refining bauxite into aluminium, producing chlorine and caustic soda from table salt, etc.
What is the Difference Between Molten and Aqueous Electrolysis?
Molten and aqueous electrolysis are analytical techniques in chemistry that are useful in separating chemical elements in the analyte substance. The key difference between molten and aqueous electrolysis is that molten electrolysis produces elements of the analyte, whereas aqueous electrolysis produces an aqueous salt solution and a mixture of gases as the final product.
Below infographic shows more comparisons related to the difference between molten and aqueous electrolysis.
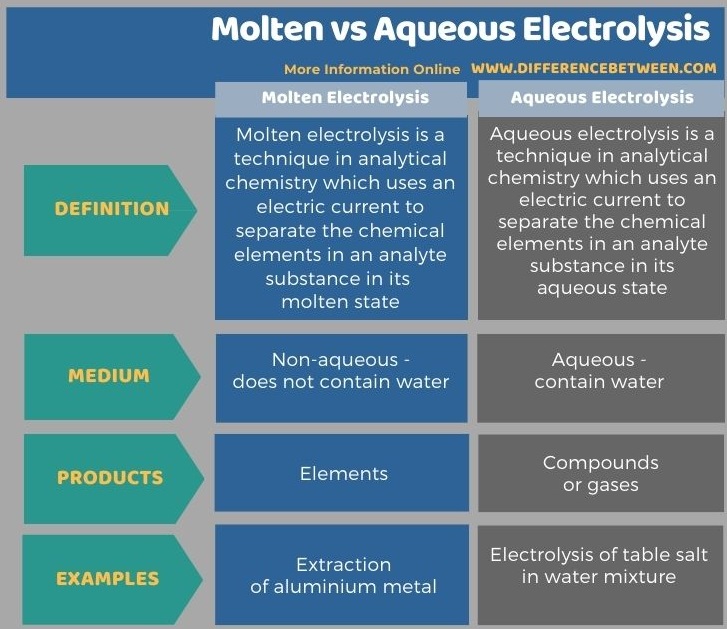
Summary – Molten vs Aqueous Electrolysis
Electrolysis is a technique in analytical chemistry which includes the use of electricity to separate elements in a substance. Molten and aqueous electrolysis are two such types of electrolysis. The key difference between molten and aqueous electrolysis is that molten electrolysis produces elements of the analyte, whereas aqueous electrolysis produces an aqueous salt solution and a mixture of gases as the final product.
Reference:
1. “Electrolysis of Molten Compounds.” Study.com, Available here.
Image Courtesy:
1. “Mg by electrolysis” By Raveendra R.S. – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Water electrolysis NaCl” By Slower – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265zqWrnqZdlrulecCqrJ6npah6prjEnKurp5yuwKq%2Fjg%3D%3D