Key Difference – Methane vs Ethane
Methane and Ethane are the smallest members of the alkane family. The molecular formulas of these two organic compounds are CH4 and C2H6 respectively. The key difference between Methane and Ethane is their chemical structure; an Ethane molecule can be considered as two methyl groups joined as a dimer of methyl groups. The other chemical and physical differences mainly arise due to this structural difference.
What is Methane?
Methane is the smallest member of the alkane family with the chemical formula CH4 (four hydrogen atoms are bonded to one carbon atom). It is considered to be the main component of natural gas. Methane is a colourless, odorless and tasteless gas; also known as carbane, marsh gas, natural gas, carbon tetrahydride, and hydrogen carbide. It can be easily ignited, and its vapor is lighter than the air.
Methane is naturally found under the ground and under the sea floor. The atmospheric methane is considered as a greenhouse gas. Methane breaks down into CH3– with water in the atmosphere. 
What is Ethane?
Ethane is a colourless, odorless gaseous compound at standard temperature and pressure. Its molecular formula and molecular weight are C2H6 and 30.07 g·mol−1 respectively. It is isolated from natural gas, as a byproduct from petroleum refining process. Ethane is very important in ethylene production. 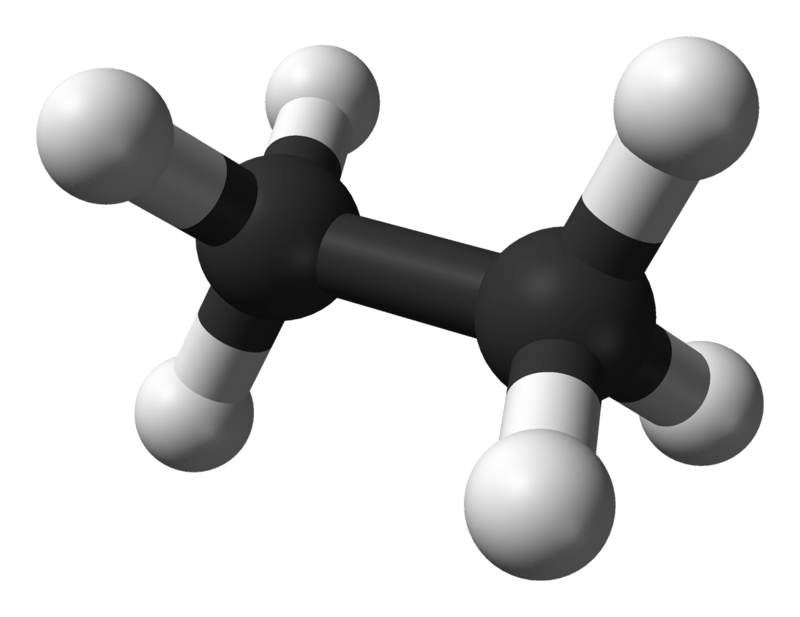
What is the difference between Methane and Ethane?
Characteristics of Methane and Ethane
Structure:
Methane: The molecular formula of methane is CH4, and it is an example of a tetrahedral molecule with four equivalent C–H bonds (sigma bonds). Bond angle between H-C-H atoms is 109.50 and all the C-H bonds are equivalent, and it is equal to108.70 pm.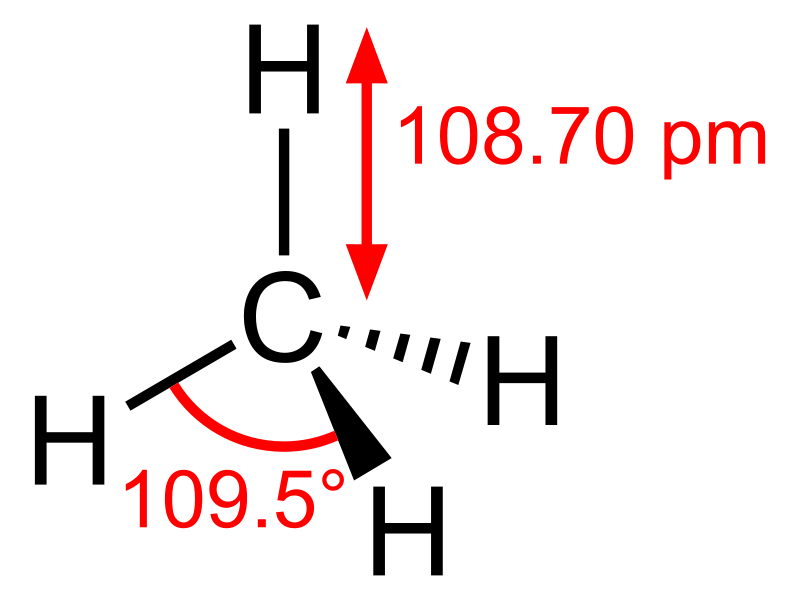
Ethane: The molecular formula of ethane is C2H6, and it is a saturated hydrocarbon since it does not contain multiple bonds.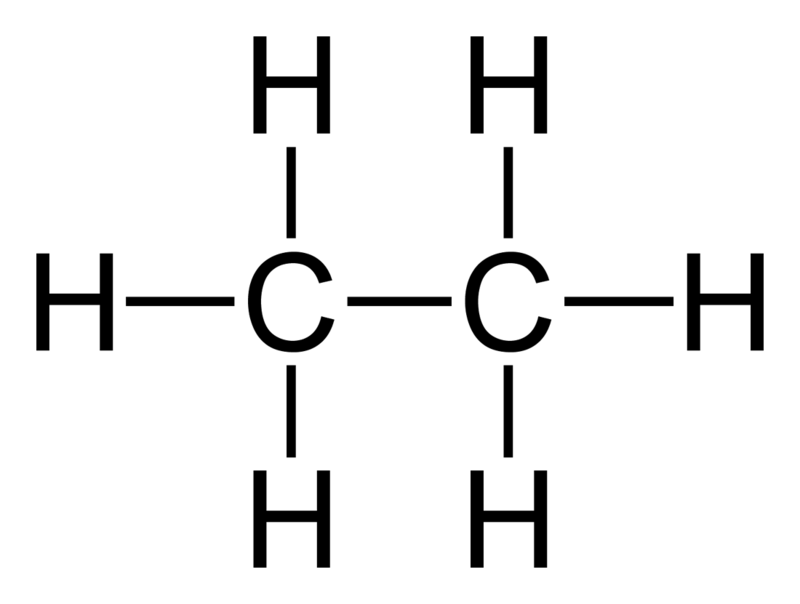
Chemical Properties:
Methane:
Stability: Methane is a chemically very stable molecule which does not react with KMnO4, K2Cr2O7, H2SO4 or HNO3 under normal conditions.
Combustion: In the presence of excess air or oxygen, methane burns with a pale-blue non-luminous flame producing carbon dioxide and water. It is a highly exothermic reaction; therefore, it is used as an excellent fuel. In the presence of insufficient air or oxygen, it partially burns into carbon monoxide (CO) gas.
Substitution Reactions: Methane shows substitution reactions with halogens. In these reactions, one or more hydrogen atoms are replaced by an equal number of halogen atoms and it is called “halogenation.” It reacts with chlorine (Cl) and bromine (Br) in the presence of sunlight.
Reaction with Steam: When a mixture of methane and steam is passed through a heated (1000 K) nickel supported on alumina surface, it can produce hydrogen.
Pyrolysis: When methane is heated to about 1300 K, it gets decomposed to carbon black and hydrogen.
Ethane:
Reactions: Ethane gas (CH3CH3) reacts with bromine vapour in the presence of light to form bromoethane, (CH3CH2Br) and hydrogen bromide (HBr). It is a substitution reaction; a hydrogen atom in ethane is substituted by bromine atom.
CH3CH3 + Br2 à CH3CH2Br + HBr
Combustion: The complete combustion of ethane produces 1559.7 kJ/mol (51.9 kJ/g) of heat, carbon dioxide, and water.
2 C2H6 + 7 O2 → 4 CO2 + 6 H2O + 3120 kJ
It can also occur without an excess of oxygen, producing a mix of amorphous carbon and carbon monoxide.
2 C2H6 + 3 O2 → 4 C + 6 H2O + energy
2 C2H6 + 5 O2 → 4 CO + 6 H2O + energy
2 C2H6 + 4 O2 → 2 C + 2 CO + 6 H2O + energy etc.
Definitions:
Substitution reactions: Substitution reaction is a chemical reaction which involves the displacement of one functional group in a chemical compound and replaced it by another functional group.
Uses:
Methane: Methane is used in many industrial chemical processes (as a fuel, natural gas, liquefied natural gas) and it is transported as a refrigerated fluid.
Ethane: Ethane is used as a fuel for motors and as a refrigerant for an extremely low-temperature system. It is shipped in steel cylinders as a liquefied gas under its own vapor pressure.
References: “Ethane”. Wikipedia. N.p., 2016. Web. 7 June 2016. Khanna, Bhishm. “What Are The Chemical Properties Of Methane ?”. Preservearticles.com. N.p., 2016. Web. 7 June 2016. “Methane | CH4 – Pubchem”. Pubchem.ncbi.nlm.nih.gov. N.p., 2016. Web. 7 June 2016. “Methane”. Wikipedia. N.p., 2016. Web. 7 June 2016. Image Courtesy: “Ball-and-stick model of methane molecule” by (Public Domain) via Commons Wikimedia “Ball-and-stick model of ethane molecule”y Ben Mills – Own work (Public Domain) via Commons Wikimedia “Methane” By Jynto – Own work, based on File:Methane-CRC-MW-dimensions-2D.png, (Public Domain) via Commons Wikimedia “Ethane” (Public Domain) via Commons WikimediancG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265xK2fmqaVYq6vsIyvqmadpJ2ur7GO