The key difference between metal and nonmetal oxides is that the metal oxides are basic compounds whereas the nonmetal oxides are acidic compounds.
The “oxides” is a large group of compounds that have chemical elements bound essentially to oxygen atoms. However, noble gases do not form these compounds due to their inert nature and higher stability. Most of the metals and nonmetals form oxides with different oxidation states while some other chemical elements form oxides with a fixed oxidation state; for example, magnesium forms only the magnesium oxide having the chemical formula MgO while Vanadium forms various oxides such as V2O3 and V2O5.
CONTENTS
1. Overview and Key Difference
2. What are Metal Oxides
3. What are Nonmetal Oxides
4. Side by Side Comparison – Metal vs Nonmetal Oxides in Tabular Form
5. Summary
Metal oxides are inorganic chemical compounds containing metals bound essentially with oxygen atoms. In these compounds, oxygen is essentially the anion of the compound having the -2 oxidation state. Therefore, metal is the cation of the compound. The metals that form oxides are in the alkali metal group (group 1 elements), alkaline earth metals (group 2 elements) and d block elements including transition metals. They form an ionic oxide, meaning, the oxide compounds they form have an ionic nature. But some chemical elements form oxides with a covalent nature, especially the chemical elements showing higher oxidation states.

Figure 01: Silver(II) Oxide
Most of the times, metal oxides are crystalline solids and are often basic compounds. Therefore, they can react with water to give an alkaline solution. Moreover, they can react with acids to form salts via neutralization reactions. Although almost all the oxides have oxygen with -2 oxidation state, there can be oxides with -1 and -1/2 oxidation states; we call them peroxides and superoxides respectively. The number of oxygen atoms in the compounds depends on the oxidation state of the metal.
Examples for metal oxides:
- Sodium oxide (Na2O)
- Magnesium oxide (MgO)
- Vanadium pentoxide (V2O5)
- Silver oxide (AgO)
Nonmetal oxides are inorganic chemical compounds containing nonmetals bound essentially with oxygen atoms. Therefore, these compounds mainly contain p block elements because p block elements are the nonmetals we have. Almost all the nonmetal oxides are covalent compounds because they tend to share electrons with other atoms, here, with oxygen atoms.
These are acidic compounds; hence they form an acid when dissolved in water. Due to the same reason, they can react with bases to form salts via neutralization reactions. Moreover, they can form oxyacids which can form hydroxides in an aqueous medium.

Figure 02: Quart or Silicone Dioxide is a Nonmetal Oxide
Examples for nonmetal oxides:
- Sulfur dioxide (SO2) and sulfur trioxide (SO3)
- Carbon dioxide (CO2) and carbon monoxide (CO)
- Silicone dioxide (SiO2)
- Nitrogen oxides (N2O, NO2, N2O5)
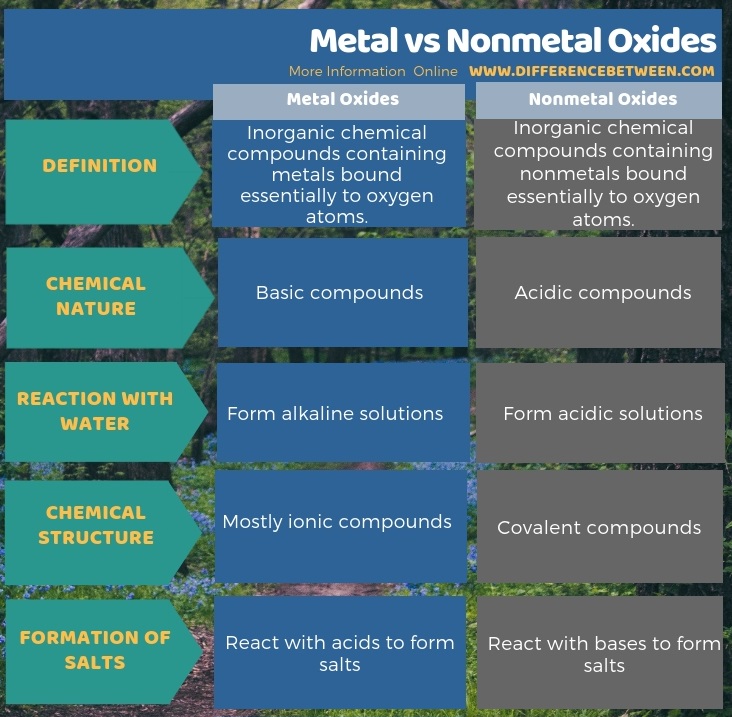
Metal oxides are inorganic chemical compounds containing metals bound essentially with oxygen atoms whereas nonmetal oxides are inorganic chemical compounds containing nonmetals bound essentially with oxygen atoms. This is the fundamental difference between metal and nonmetal oxides. Moreover, these compounds differ from each other according to their chemical nature. Thus, the key difference between metal and nonmetal oxides is that the metal oxides are basic compounds whereas the nonmetal oxides are acidic compounds.
Moreover, there are some difference between metal and nonmetal oxides in their chemical structure as well. Most of the time, metal oxides are ionic compounds while nonmetal oxides are covalent compounds. Also, metal oxides tend to react with water to form alkaline solutions but, the nonmetal oxides tend to react with water to form acidic solutions. This is another significant difference between metal and nonmetal oxides. Furthermore, the metal oxides react with acids to form salts whereas, the nonmetal oxides react with bases to form salts.
Summary – Metal vs Nonmetal Oxides
Oxides are chemical compounds having either a metal or a nonmetal bound with one or more oxygen atoms. The key difference between metal and nonmetal oxides is that the metal oxides are basic compounds whereas the nonmetal oxides are acidic compounds.
Reference:
1. Zumdahl, Steven S. “Oxide.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 7 May 2018. Available here
Image Courtesy:
1.”Silver(I)-oxide-sample”By Benjah-bmm27 – Own work, (Public Domain) via Commons Wikimedia
2.”Quartz oisan”By Didier Descouens – Own work, (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265xK2YpWWRo7Fuus6npJ6skaF6sMTInZysZw%3D%3D