The key difference between maleic acid and fumaric acid is that maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer.
Maleic acid and fumaric acid are carboxylic acids. They are cis-trans isomers of each other. Both these compounds have two carboxylic acid groups per molecule.
CONTENTS
1. Overview and Key Difference
2. What is Maleic Acid
3. What is Fumaric Acid
4. Side by Side Comparison – Maleic Acid vs Fumaric Acid in Tabular Form
5. Summary
What is Maleic Acid?
Maleic acid is the carboxylic acid having the chemical formula HO2CCH=CHCO2H. It is a dicarboxylic acid because it has two carboxylic groups per molecule. It is an isomer of fumaric acid. The molar mass of maleic acid is 116.072 g/mol. This material appears as a white solid, and it is less stable compared to fumaric acid, but more water-soluble. Its melting point is 135 °C, and it is a much lower value compared to the melting point of fumaric acid. Above this temperature, the compound decomposes. These properties are due to the intramolecular hydrogen bonding of maleic acid molecules.
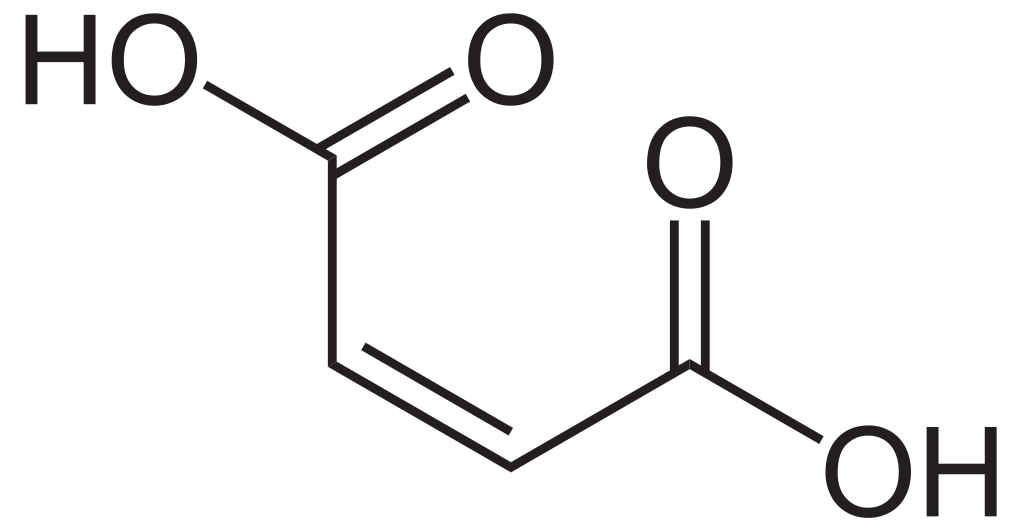
Figure 01: Structure of Maleic Acid
In the industrial scale, we produce maleic acid via hydrolysis of maleic anhydride. We can also produce it using the oxidation of benzene or butane.
What is Fumaric Acid?
Fumaric acid is the carboxylic acid having the chemical formula HO2CCH=CHCO2H. Further, this compound has a fruity taste; thus, we can use it as a food additive. The molar mass of the compound is 116.072 g/mol. It is equal to the molar mass of maleic acid since both have the same chemical formula.
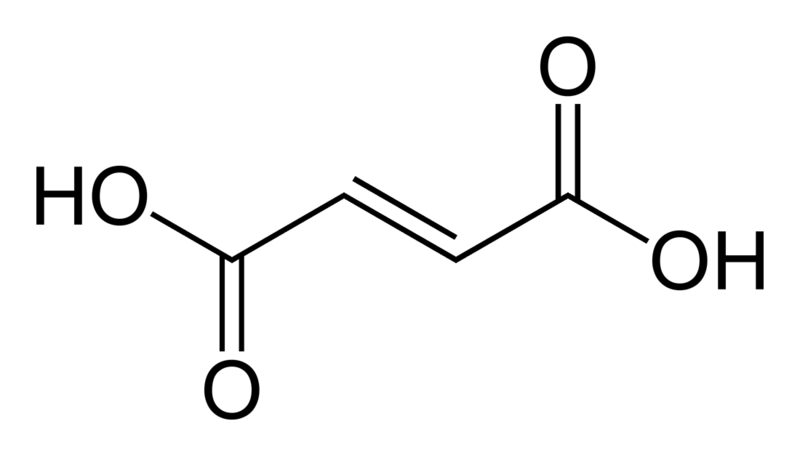
Figure 02: Structure of Fumaric Acid
Moreover, this compound appears as a white solid. The melting point is 287 °C, and upon further heating, the compound decomposes. Besides, we can produce fumaric acid via catalytic isomerization of maleic acid at low pH. Also, this is done in an aqueous solution.
What is the Difference Between Maleic Acid and Fumaric Acid?
The key difference between maleic acid and fumaric acid is that maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer. Moreover, maleic acid forms weak intramolecular hydrogen bonds and has a much lower melting point than fumaric acid. It is because the intramolecular hydrogen bonds in fumaric acid are much stronger due to the trans geometry.
Below infographic provides more details on the difference between maleic acid and fumaric acid.
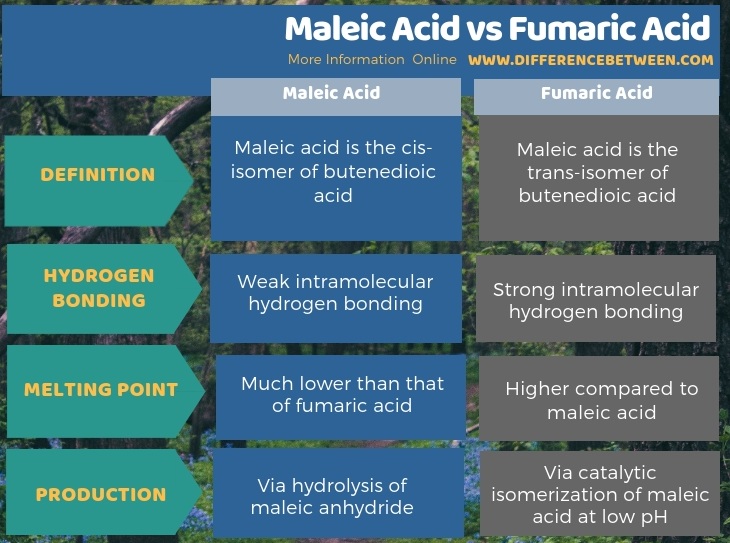
Summary – Maleic Acid vs Fumaric Acid
Both maleic acid and fumaric acid are carboxylic acids. Moreover, they are cis-trans isomers of each other. The key difference between maleic acid and fumaric acid is that maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer.
Reference:
1.“Maleic Acid.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, Available here.
Image Courtesy:
1. “Maleinsäure” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
2. “Fumaric-acid-2D-skeletal” By Ben Mills – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265wKWcoptdlrCqsIyapZ1llqq6or7InGSam5mZfA%3D%3D