The key difference between LiAlH4 and NaBH4 is that LiAlH4 can reduce esters, amides and carboxylic acids whereas NaBH4 cannot reduce them.
Both LiAlH4 and NaBH4 are reducing agents. But LiAlH4 is a very strong reducing agent than NaBH4 because the Al-H bond in the LiAlH4 is weaker than the B-H bond in NaBH4. This makes the Al-H bond less stable. The reason for this is the low electronegativity of Aluminum compared to Boron. Therefore, the low electronegativity shifts the electron density towards the hydrogen in Al-H than that of B-H bond. As a result, LiAlH4 is a better hydride donor.
CONTENTS
1. Overview and Key Difference
2. What is LiAlH4
3. What is NaBH4
4. Side by Side Comparison – LiAlH4 vs NaBH4 in Tabular Form
5. Summary
What is LiAlH4?
LiAlH4 is lithium aluminium hydride, which is a strong reducing agent. The scientists Finholt, Bond and Schlesinger first discovered this compound in 1947. Furthermore, there are many applications of this compound in organic synthesis processes. It is dangerously reactive towards water, which leads to release gaseous hydrogen (H2).
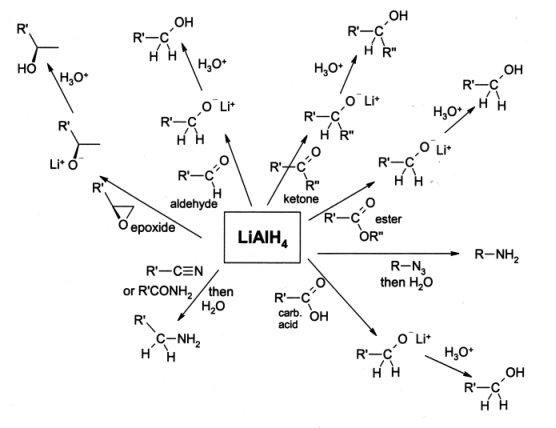
Figure 01: Reducing Power of LiAlH4
It appears as white crystals in pure form. But the commercial grade LiAlH4 is a grey colored powder due to contaminations. This solid compound is highly hygroscopic and odorless. The molar mass is 37.95 g/mol, and the melting point is 150◦C. In order to purify this material, we can use a recrystallisation method with diethyl ether.
What is NaBH4?
NaBH4 is sodium borohydride, which is a reducing agent. Unlike LiAlH4, this is a weak reducing agent. It appears as white crystals that are highly hygroscopic. Moreover, this compound is soluble in water and also reacts with water. However, it slowly hydrolyzes in water.
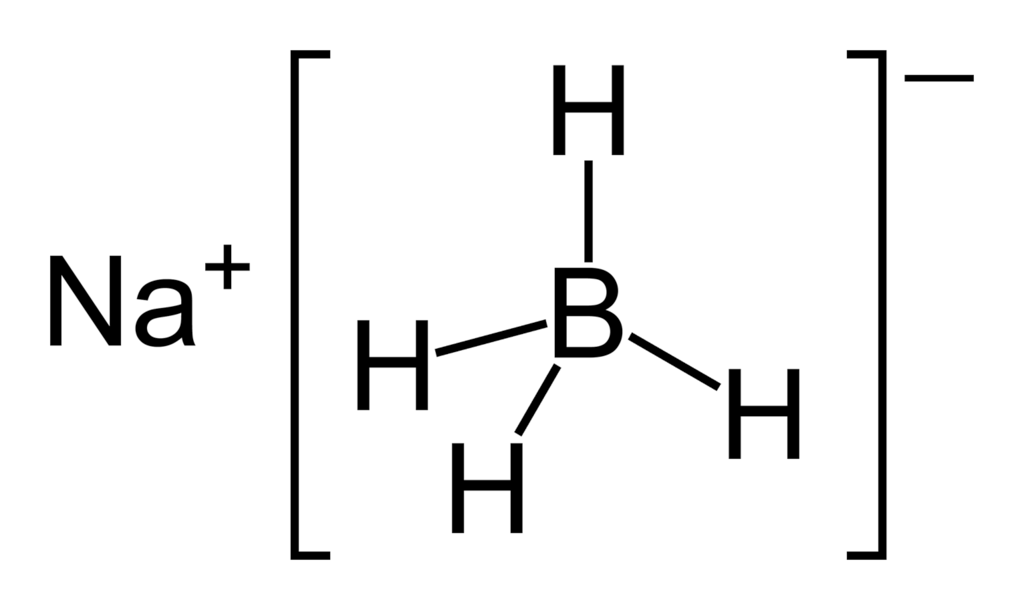
Figure 02: Structure of Sodium Borohydride
The molar mass of this compound is 37.83 g/mol, and the melting point is 400◦C. At higher temperatures, it tends to decompose. The NABH4 powder often tends to form lumps. In order to purify this compound, we can use recrystallisation methods with warm diglyme. Although this compound decomposes in neutral or acidic mediums, it is stable at pH 14. The compounds that NaBH4 can reduce include organic carbonyls such as aldehydes and ketones, acyl chlorides, thiol esters, imines, etc.
What is the Difference Between LiAlH4 and NaBH4?
LiAlH4 is lithium aluminium hydride which is a strong reducing agent. Its molar mass is 37.95 g/mol. It is a very strong reducing agent when compared to NaBH4 since this compound can reduce even esters, amides and carboxylic acids. This is the main difference between LiAlH4 and NaBH4.
NaBH4 is sodium borohydride, which is also a reducing agent. But, it is a mild reducing agent which cannot reduce esters, amides and carboxylic acids. Its molar mass is 37.83 g/mol.
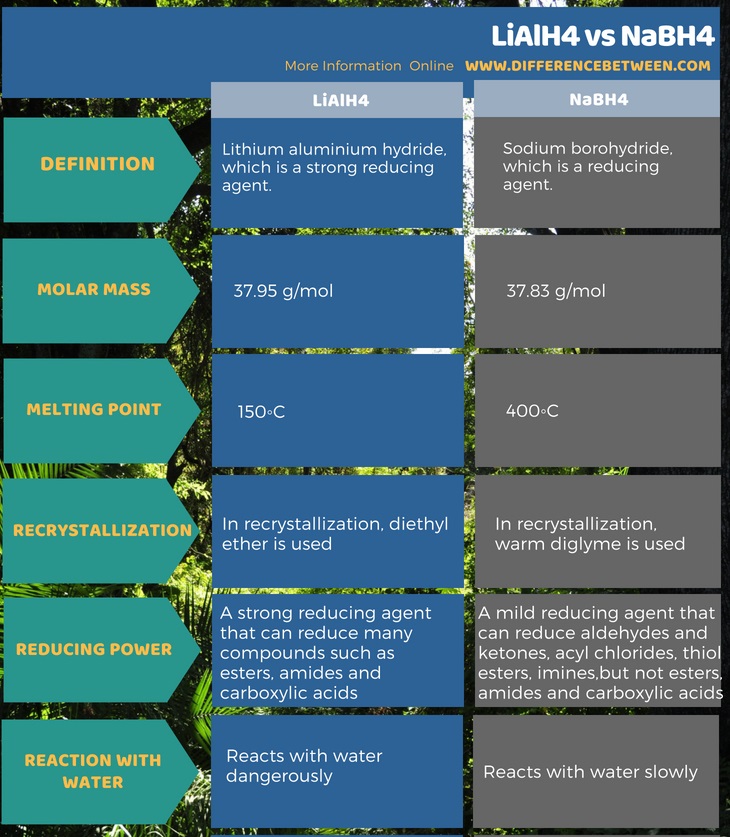
Summary – LiAlH4 vs NaBH4
Both LiAlH4 and NaBH4 are important reducing agents in organic synthesis mechanisms. The difference between LiAlH4 and NaBH4 is that LiAlH4 can reduce esters, amides and carboxylic acids whereas NaBH4 cannot reduce them.
Reference:
1. “Lithium Aluminium Hydride.” Wikipedia, Wikimedia Foundation, 7 June 2018. Available here
2. “Sodium Borohydride.” Wikipedia, Wikimedia Foundation, 7 June 2018. Available here
Image Courtesy:
1.’LAH rxns’ (Public Domain) via Commons Wikimedia
2.’Sodium-borohydride (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau264yJqjoWxdlrulec2amaFsXw%3D%3D