The key difference between left and right handed amino acids is that the amine groups of left-handed amino acid occur in the left-hand side of the molecule whereas the amine group of right handed amino acids is in the right-hand side.
Chirality is an important phenomenon in organic chemistry. It describes the presence of a carbon atom, which has four different groups attached to it. That means; a chiral compound has an asymmetric carbon centre. Left-handed and right handed amino acids are two types of organic compounds that have chiral centres.
CONTENTS
1. Overview and Key Difference
2. What is Left-Handed Amino Acids
3. What is Right Handed Amino Acids
4. Side by Side Comparison – Left vs Right Handed Amino Acids in Tabular Form
5. Summary
What is Left Handed Amino Acids?
Left handed amino acids are the stereoisomers in which the amine group of the molecule exists in the left-hand side. We also call them as L-amino acids as well. When considering the general structure, this type of amino acid molecules has a central chiral carbon, a hydrogen atom attached to this carbon at the top, alkyl group attached at the bottom, amine group at the left-hand side and the carboxylic group at the right-hand side.
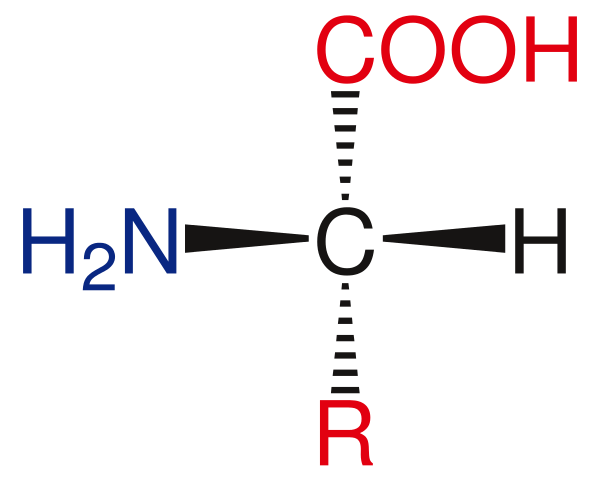
Figure 01: General Structure of Left-Handed Amino Acid
These compounds occur in all proteins of animals, plants, fungi, etc. Moreover, cells use them to produce proteins. When considering their role in biological systems, they can act as enzymes, like hormones, etc.
What is Right Handed Amino Acids?
Right-handed amino acids are the stereoisomers in which the amine group of the molecule exists in the right-hand side. Moreover, we can call them D-amino acids. When considering the general structure of these molecules, there is a central chiral carbon atom attached with a hydrogen atom at the top, an alkyl group at the bottom, amine group at the right-hand side and a carboxylic acid group at the left-hand side.
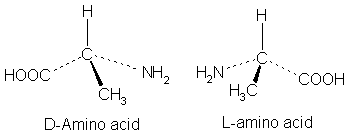
Figure 02: L (Left Handed) and D (Right Handed) Amino Acids
Usually, there are no right handed amino acid molecules incorporated into proteins by cells. However, some of them occur in peptidoglycan cell walls of bacteria. In addition to that, some of these compounds (i.e. D-serine) acts as a neurotransmitter in our brain.
What is the Difference Between Left and Right Handed Amino Acids?
Left-handed amino acids are the stereoisomers in which the amine group of the molecule exists in the left-hand side while right handed amino acids are the stereoisomers in which the amine group of the molecule exists in the right-hand side. Therefore, this is the key difference between left and right handed amino acids,
Moreover, another significant difference between left and right handed amino acids is that the left-handed amino acid has a central chiral carbon, a hydrogen atom at the top, an alkyl group at the bottom, amine group at left hand side and the carboxylic group at the right hand side. But right handed amino acid has a central chiral carbon, a hydrogen atom at the top, an alkyl group at the bottom, amine group at right-hand side and the carboxylic group at the left-hand side.
The below infographic on the difference between left and right handed amino acids presents these differences in tabular form.

Summary – Left vs Right Handed Amino Acids
Both left and right handed amino acids are very useful for cells in different functions. In summary, the key difference between left and right handed amino acids is that the amine groups of left handed amino acid occur in the left-hand side of the molecule whereas the amine group of right handed amino acids is in the right-hand side.
Reference:
1. “Chirality (Chemistry).” Wikipedia, Wikimedia Foundation, 21 Dec. 2018. Available here
Image Courtesy:
1.”L-amino acid general”By Leyo – Own work, (Public Domain) via Commons Wikimedia
2.”Op isomer” (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau264xJ%2BrZpmemXqztcahq2agkaOxprCMmqSipp9irqS1w6xm