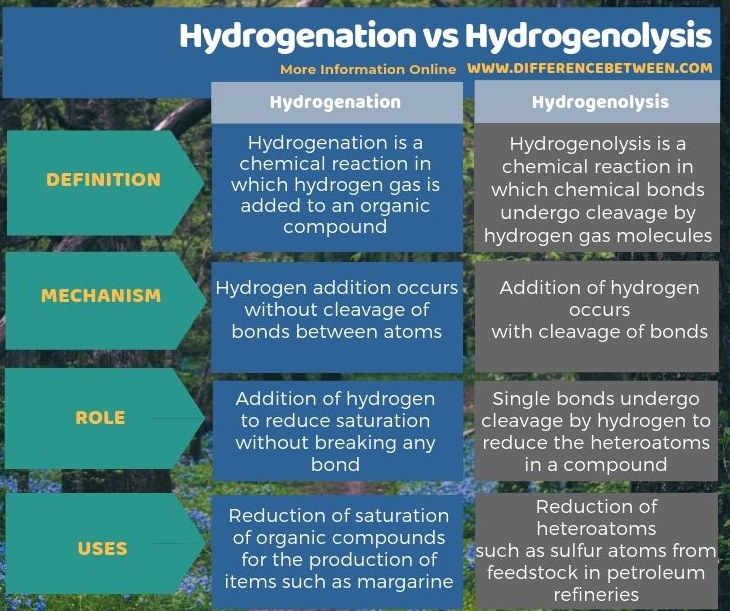
The key difference between hydrogenation and hydrogenolysis is that hydrogenation includes the addition of hydrogen without cleavage of bonds, whereas hydrogenolysis includes the addition of hydrogen with the cleavage of bonds.
Both hydrogenation and hydrogenolysis are important industrial processes in the food industry and the petrochemical industry.
CONTENTS
1. Overview and Key Difference
2. What is Hydrogenation
3. What is Hydrogenolysis
4. Side by Side Comparison – Hydrogenation vs Hydrogenolysis in Tabular Form
5. Summary
What is Hydrogenation?
Hydrogenation is a chemical reaction where hydrogen gas is added to an organic compound. Further, this process mainly involves bond formation. Also, this reaction usually requires a catalyst such as nickel, platinum or palladium. Moreover, the hydrogenation process is very important in saturating organic compounds.
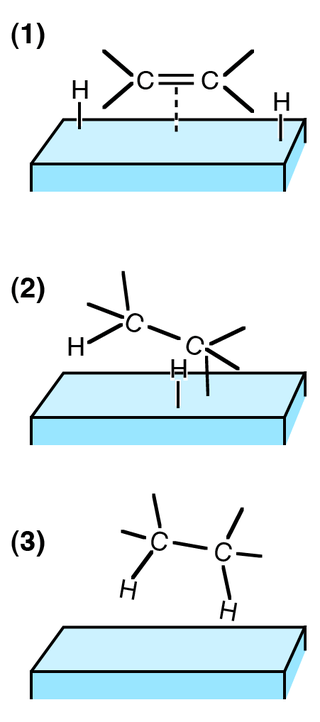
Figure 01: Process of Hydrogenation in Three Steps
Furthermore, the reaction mechanism includes the addition of pairs of hydrogen atoms to a molecule on the surface of the catalyst. Generally, the reactants for this reaction are alkenes (unsaturated compounds having double bonds between carbon atoms). Moreover, for a stable hydrogenation reaction, we need catalysts. However, if there is no catalyst, we need to provide very high temperatures for the reaction to proceed.
What is Hydrogenolysis?
Hydrogenolysis is a chemical reaction in which chemical bonds undergo cleavage by hydrogen gas molecules. Further, this process includes the cleavage of both C-C bonds and C-heteroatom bonds.
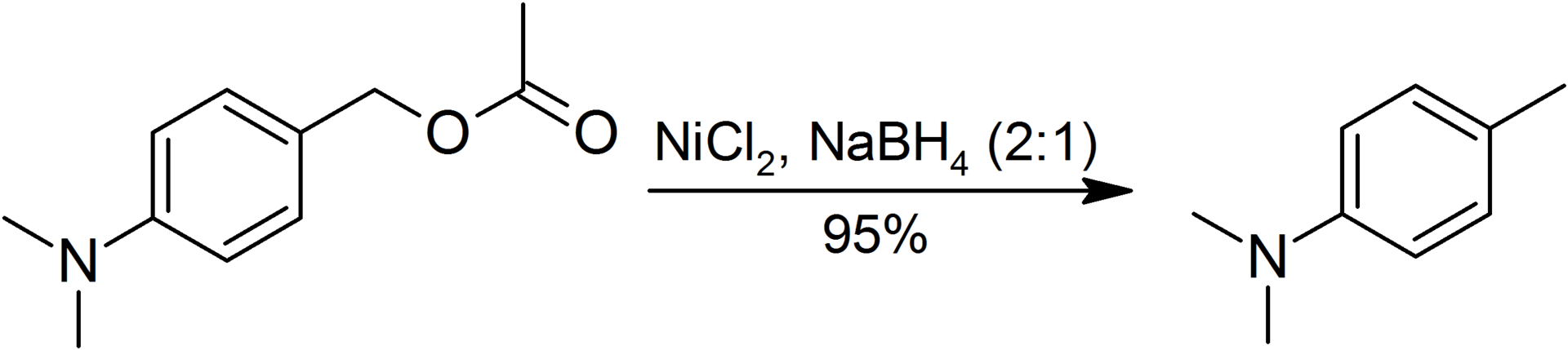
Figure 02: Hydrogenolysis of Benzyl Ester
Here, the heteroatom is usually oxygen, sulfur, nitrogen etc. This reaction also requires catalysts such as palladium, Raney metals such as Raney nickel, etc.
What is the Difference Between Hydrogenation and Hydrogenolysis?
The key difference between hydrogenation and hydrogenolysis is that hydrogenation includes the addition of hydrogen without cleavage of bonds, whereas hydrogenolysis includes the addition of hydrogen with the cleavage of bonds. Hydrogenation occurs via the addition of hydrogen to reduce saturation without breaking any bond while hydrogenolysis occurs via single bonds undergo cleavage by hydrogen to reduce the heteroatoms in a compound.
Below infographic shows more information on the difference between hydrogenation and hydrogenolysis.
Summary – Hydrogenation vs Hydrogenolysis
The key difference between hydrogenation and hydrogenolysis is that hydrogenation includes the addition of hydrogen without cleavage of bonds, whereas hydrogenolysis includes the addition of hydrogen with the cleavage of bonds.
Reference:
1. Morales-Delarosa, S., and J.m. Campos-Martin. “Catalytic Processes and Catalyst Development in Biorefining.” Advances in Biorefineries, 2014, pp. 152–198., doi:10.1533/9780857097385.1.152.
Image Courtesy:
1. “Hydrogenation on catalyst” By Michael Schmid – Drawing created myself (CC BY 1.0) via Commons Wikimedia
2. “Hydrogenolysis of a benzylic ester by Nickel boride” By LHcheM – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2602J2pqJ%2BVo661tc6nZJqmlGK1urDRqJ6epp%2BhxrS10mg%3D