The key difference between heat capacity and specific heat is that heat capacity is dependent on the amount of substance, while specific heat capacity is independent of it.
When we heat a substance, its temperature rises, and when we cool it, its temperature decreases. This difference in temperature is proportional to the amount of heat supplied. Heat capacity and specific heat are two proportionality constants that relate to the temperature change and the amount of heat.
CONTENTS
1. Overview and Key Difference
2. What is Heat Capacity
3. What is Specific Heat
4. Side by Side Comparison – Heat Capacity vs Specific Heat in Tabular Form
5. Summary
What is Heat Capacity?
In thermodynamics, the total energy of a system is the internal energy. Internal energy specifies the total kinetic and potential energy of molecules in the system. We can change the internal energy of a system either by doing work on the system or by heating it. The internal energy of a substance increases when we increase its temperature. The amount of increase depends on the conditions at which heating takes place. Here, we need heat to increase the temperature.
Heat capacity (C) of a substance is “the quantity of heat needed to raise the temperature of a substance by one degree Celsius (or one kelvin).” Heat capacity differs from substance to substance. The amount of substance is directly proportional to the heat capacity. That means by doubling the mass of a substance, the heat capacity becomes doubled. The heat we need to increase the temperature from t1 to t2 of a substance can be calculated using the following equation.
q= C x ∆t
q= required heat
∆t= t1-t2
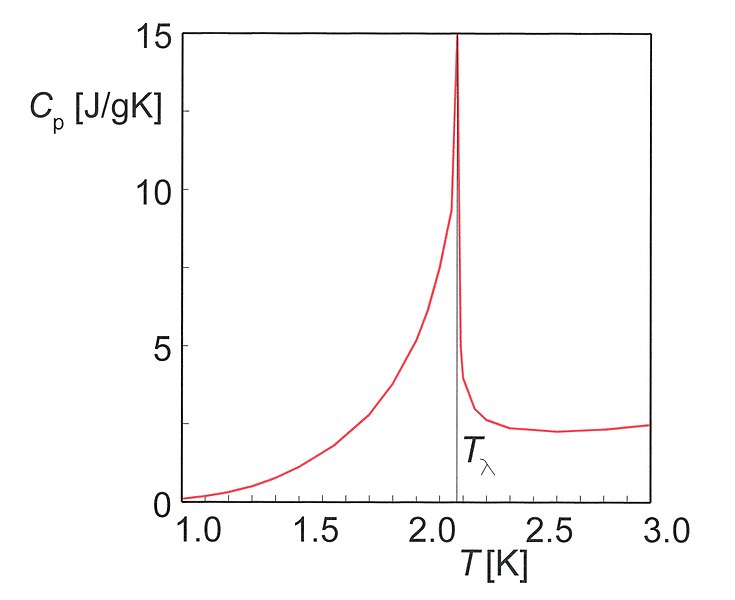
Figure 01: Heat Capacity of Helium
The unit of heat capacity is JºC-1 or JK-1. Two types of heat capacities are defined in thermodynamics; heat capacity at constant pressure and heat capacity at constant volume.
What is Specific Heat?
Heat capacity depends on the amount of substance. Specific heat or specific heat capacity (s) is the heat capacity which is independent of the amount of substances. We can define it as “the quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one Kelvin) at a constant pressure.”
The unit of specific heat is Jg-1oC-1. The specific heat of water is very high, with a value of 4.186 Jg-1oC-1. This means, to increase the temperature of 1 g of water by 1°C, we need 4.186 J of heat energy. This high value accounts for the role of water in thermal regulation. To find the heat needed to increase the temperature of a certain mass of a substance from t1 to t2, the following equation can be used.
q= m x s x ∆t
q= required heat
m= mass of the substance
∆t= t1-t2
However, the above equation does not apply if the reaction involves a phase change; for example, when water goes to a gas phase (at the boiling point), or when water freezes to form ice (at the melting point). This is because the heat added or removed during the phase change does not change the temperature.
What is the Difference Between Heat Capacity and Specific Heat?
The key difference between heat capacity and specific heat is that heat capacity is dependent on the amount of substance while specific heat capacity is independent of it. Furthermore, when considering the theory, the heat capacity of the amount of heat needed to change a substances’ temperature by 1°C or 1K while specific heat is the heat needed to change 1g of substances’ temperature by 1°C or 1K.
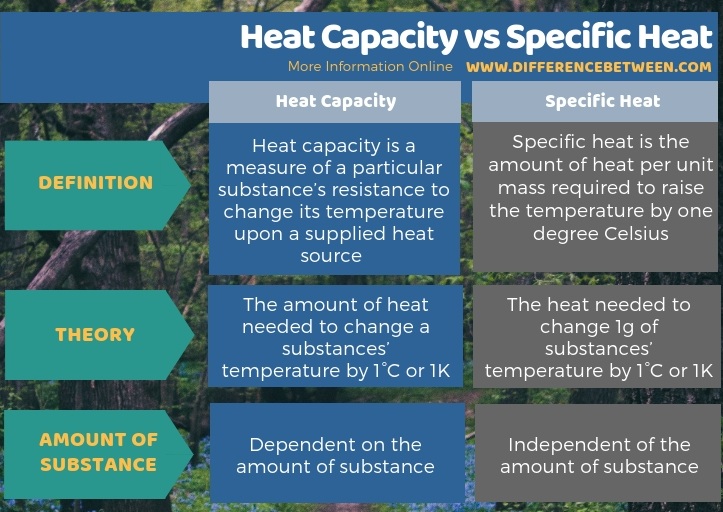
Summary – Heat Capacity vs Specific Heat
Heat capacity and specific heat are important terms in thermodynamics. The key difference between heat capacity and specific heat is that heat capacity is dependent on the amount of substance while specific heat capacity is independent of it.
Reference:
1. Helmenstine, Anne Marie. “Specific Heat Capacity in Chemistry.” ThoughtCo, Mar. 21, 2019, Available here.
Image Courtesy:
1. “Heat capacity of 4He 01” By Adwaele at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau260xJqrZpuRpa6ktdOyZJqmlGLDtHnSqZycoZaesG60xJqraA%3D%3D