Glycerine vs Glycerol
Glycerol and glycerin are two terms that are confusing to many people and used interchangeably. Most of the time, both have the same usages. Although they seem to be the same, there is a difference between the two terms. Commonly we use the term glycerin, which is the commercial term for the compound glycerol, and there are few other differences between the two.
Glycerol
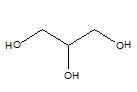
Glycerol is a polyol molecule with the molecular formula HOCH2CHOHCH2OH. According to the IUPAC nomenclature, glycerol is named as Propan-1, 2, 3-triol. Its molar mass is 92.09 g mol−1. It has three –OH groups attached to three separate carbon atoms. This belongs to the alcohol family in organic chemistry. It is a viscous, colorless liquid. Further it is odorless and sweet in taste. The structure of glycerol is as follows.
Because of the three-hydroxyl groups, glycerol molecule is highly polar. This makes them highly soluble in water and other polar solvents. Glycerol forms lipid with the combination of three fatty acids. The –OH group of glycerol and -COOH groups of fatty acids make ester bonds, and produce a triglyceride. So glycerol is the backbone of a triglyceride. Since triglycerides are the compounds in soap, glycerol is useful in making soap. Moreover, this is widely used in pharmaceutical applications. It is used as a tablet-binding agent, to provide lubrication and as a laxative. Glycerol is a treatment for burns, bites, cuts, and psoriasis. Glycerol is humectants; therefore, it is used in moisturizers. Other than this, glycerol is used as an ingredient in our day-to-day used products like toothpaste, shaving cream, hair care products, mouthwashes, etc. In the food industry, this is used as a sweetener and solvent, and used to preserve food. Glycerol is a sugar alcohol, so it is used in food instead of sugar to give the sweet taste. It has low calories compared to sugar (27 calories per tea spoon), so it is a good alternative for sugar. Glycerol is used to produce gun powder and various explosives. Nitroglycerin is an explosive material which is produced using glycerol.
Glycerine
This is a commercial term. When there is more than 95% glycerol in a product, it is known as glycerin. Though the chemical term for such a sample should be glycerol, for the usage glycerine is commonly used. However, glycerol is the chemical term which shows the exact compound in the sample. Glycerin is used for most of the uses as states above under glycerol. But since, glycerin doesn’t contain pure glycerol; it cannot be used for some of the purpose where pure glycerol is needed. For example, when treating cuts and burns, pure glycerol is needed.
What is the difference between Glycerin and Glycerol? • Glycerin is the commercial term for a sample containing more than 95% glycerin. • Therefore, glycerin does not purely contain glycerol. • The usages of two are quite different. Glycerol is used for medical applications, scientific purposes where pure glycerol is needed. And glycerin is used in cosmetic and day-to-day products. |
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26zy7KanqqZo7Jurc2dZK%2BrXZy5uq%2FEq6alZw%3D%3D