The key difference between functional group and substituent is that the functional group is an active part of a molecule whereas substituent is a chemical species that can replace an atom or a group of atoms in a molecule.
The terms functional group and substituent are often found in organic chemistry. A functional group is a specific type of substituent which causes the activity of a molecule. This means the reactions a certain molecule undergoes is determined by the functional group. However, a substituent can be either an active chemical species or an inactive chemical species.
CONTENTS
1. Overview and Key Difference
2. What is a Functional Group
3. What is a Substituent
4. Side by Side Comparison – Functional Group vs Substituent in Tabular Form
5. Summary
What is a Functional Group?
A functional group is a specific substituent within a molecule that is responsible for the characteristic chemical reactions of those molecules. If the functional group is the same for two molecules that have different chemical structures, the two molecules will undergo similar types of reactions, no matter the size of the molecules. The functional groups are very important in different aspects; in identifying unknown molecules, in determining end products of reactions, in chemical synthesis reactions for the designing and synthesis of new compounds, etc.
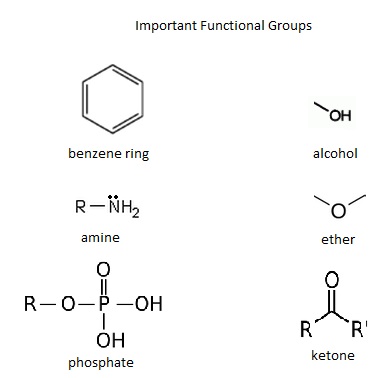
Figure 01: Some Important Functional Groups
Generally, functional groups are attached to the molecule via covalent chemical bonds. In polymer materials, the functional groups are attached to the nonpolar core of carbon atoms, giving the polymer its specific characteristic features. Sometimes, the functional groups are charged chemical species. i.e. carboxylate ion group. This makes the molecule a polyatomic ion. In addition, the functional groups that attach to a central metal atom in coordinate complexes are called ligands. Some common examples for functional groups include hydroxyl group, carbonyl group, aldehyde group, ketone group, carboxyl group, etc.
What is a Substituent?
A substituent is an atom or a group of atoms that can replace one or more atom in a molecule. Here, the substituent tends to attach with this new molecule. When considering the types of substituents, there are either active groups such as functional groups and inactive groups as well. Furthermore, steric effects may arise due to the volume occupied by the substituents in the molecule they substitute. There can also be polar effects that arise due to the combination of inductive effects and mesomeric effects. Apart from that, the terms most-substituted and least-substituted are useful when explaining the relative number of substituents in different molecules.
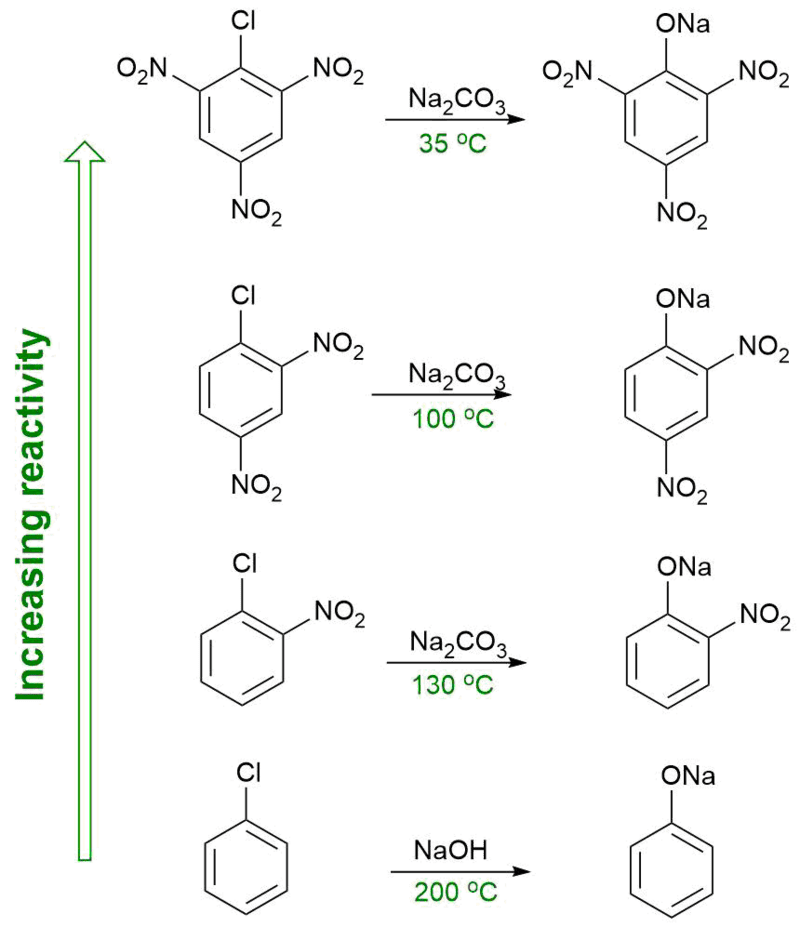
Figure 02: Placement of Substituent Groups so that the Reactivity of the Molecule Increases
When naming organic compounds, we need to consider the types of substituents they have and the positions of those substituents as well. For example, the suffix –yl means, one hydrogen atom of the molecule is replaced; -ylidene means two hydrogen atoms (by a double bond between molecule and new substituent) and –ylidyne means three hydrogen atoms are replaced by a substituent (by a triple bond between molecule and new substituent).
What is the Difference Between Functional Group and Substituent?
The key difference between functional group and substituent is that a functional group is an active part of a molecule whereas a substituent is a chemical species that can replace an atom or a group of atoms in a molecule. Furthermore, functional groups are active groups, and they cause the specific characteristics of the molecule. In fact, they are a specific type of substituents. On the other hand, substituents can be either active or inactive groups; that means, they may or may not cause the specific activity of the molecule.
Below infographic summarizes the difference between functional group and substituent.
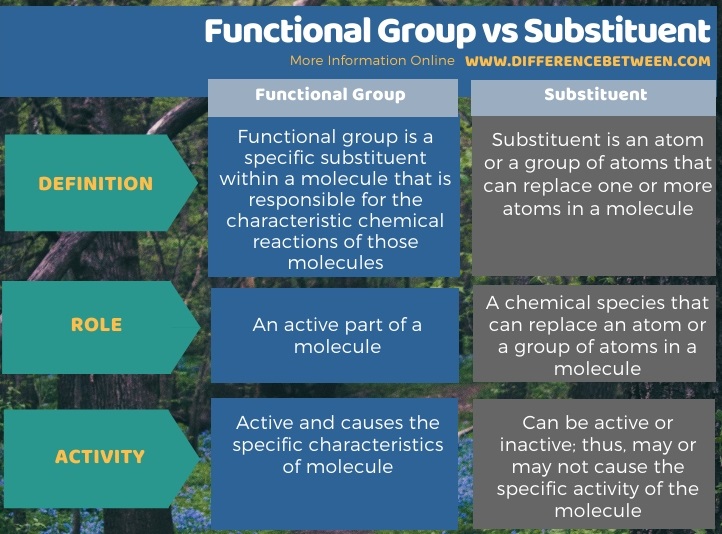
Summary – Functional Group vs Substituent
In organic chemistry, the terms functional group and substituent are often found. The key difference between functional group and substituent is that a functional group is an active part of a molecule whereas a substituent is a chemical species that can replace an atom or a group of atoms in a molecule.
Reference:
1. “4.4: Functional Groups.” Chemistry LibreTexts, Libretexts, 9 Sept. 2019, Available here.
Image Courtesy:
1. “Ochem 6 important functional groups” By Lhunter2099 – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Substituent Effect of Nitro in SNAr” By Zjnlive – Own work (CC0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26y1KearaGfo66tecarpq6oXZa7pXnSrpmsrJmpwqa602g%3D