The key difference between free energy and activation energy is that free energy is the amount of energy available for a thermodynamic system to perform thermodynamic work, whereas activation energy of a chemical reaction is the energy barrier that has to be overcome in order to obtain products from the reaction.
Free energy and activation energy are two different terms that have different applications as well. The term free energy is used regarding thermodynamic systems in physical chemistry, whereas the term activation energy is mainly used regarding chemical reactions in biochemistry.
CONTENTS
1. Overview and Key Difference
2. What is Free Energy
3. What is Activation Energy
4. Side by Side Comparison – Free Energy vs Activation Energy in Tabular Form
5. Summary
What is Free Energy?
Free energy is the amount of energy available for a thermodynamic system to perform thermodynamic work. Free energy has the dimensions of energy. The value of the free energy of a thermodynamic system is determined by the present state of the system, not by its history. There are two main types of free energy often discussed in thermodynamics: Helmholtz free energy and Gibbs free energy.
Helmholtz free energy is the energy that is available in a closed thermodynamic system to perform thermodynamic work at constant temperature and volume. Hence, the negative value of Helmholtz energy indicates the maximum work that a thermodynamic system can perform by holding its volume constant. In order to keep the volume constant, some of the total thermodynamic work is done as boundary work (to keep the boundary of the system as it is).
Gibbs free energy is the energy that is available in a closed, thermodynamic system to perform thermodynamic work at constant temperature and pressure. The volume of the system can vary. Free energy is denoted by G.
What is Activation Energy?
Activation energy of a chemical reaction is the energy barrier that has to be overcome in order to obtain products from the reaction. In other words, it is the minimum energy required for a reactant to convert into a product. It is always necessary to provide activation energy in order to start a chemical reaction.
We denote activation energy as Ea or AE; we measure it by the unit kJ/mol. Moreover, activation energy is considered as the minimum energy required to form the intermediate with the highest potential energy in a chemical reaction. Some chemical reactions have a slow progression and take place via two or more steps. Here, intermediates are formed and then rearranged to form the final product. Thus, the energy required to start that reaction is the energy required to form the intermediate with the highest potential energy.
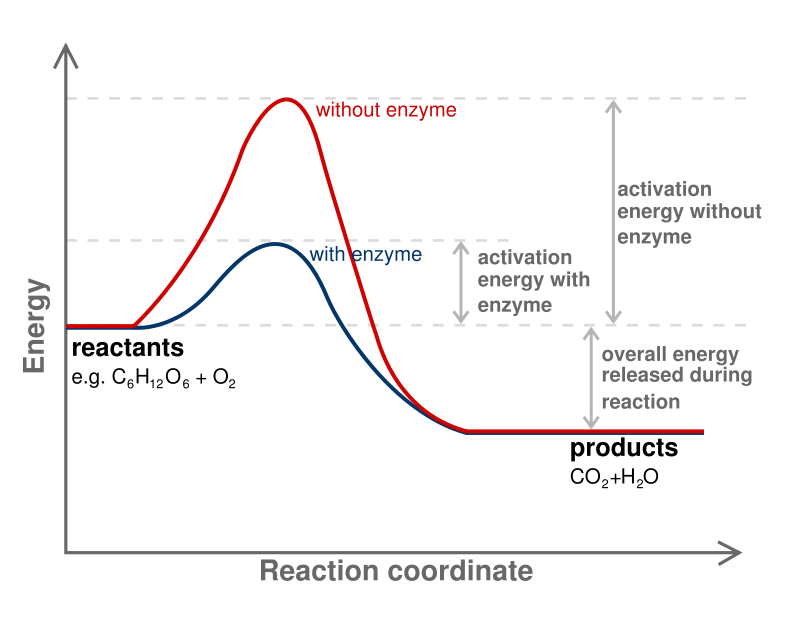
Furthermore, catalysts can reduce activation energy. Therefore, catalysts are often used in order to overcome the energy barrier and let the chemical reaction progress. Enzymes are biological catalysts that can decrease the activation energy of the reaction taking place in tissues.
What is the Difference Between Free Energy and Activation Energy?
Free energy and activation energy are two different terms that have different applications as well. The key difference between free energy and activation energy is that free energy is the amount of energy available for a thermodynamic system to perform thermodynamic work, whereas activation energy of a chemical reaction is the energy barrier that has to be overcome in order to obtain products from the reaction.
Below is the summary of the difference between free energy and activation energy in tabular form.
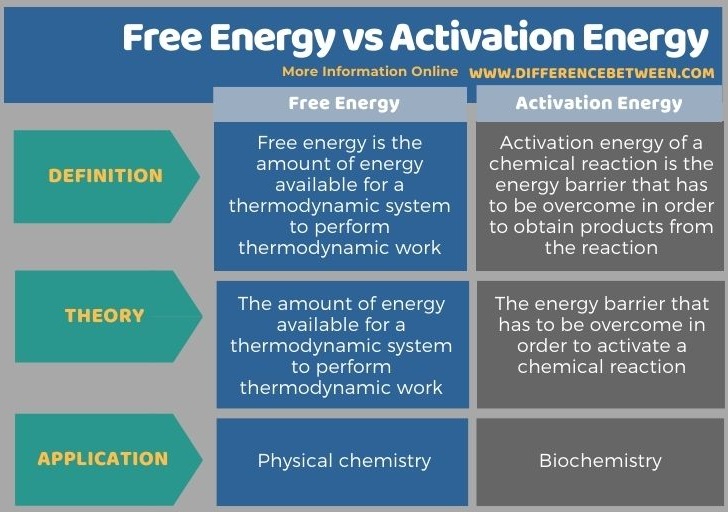
Summary – Free Energy vs Activation Energy
Free energy and activation energy are two different terms that have different applications. The key difference between free energy and activation energy is that free energy is the amount of energy available for a thermodynamic system to perform thermodynamic work, whereas activation energy of a chemical reaction is the energy barrier that has to be overcome in order to obtain products from the reaction.
Reference:
1. Helmenstine, Anne Marie. “Activation Energy Definition in Chemistry.” ThoughtCo, Aug. 27, 2020, Available here.
Image Courtesy:
1. “Activation2 updated” Originally uploaded by Jerry Crimson Mann, vectorized by Tutmosis, corrected by Fvasconcellos – en:Image:Activation2.png (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26y0Z6cZp2emr%2BoxYyapZ1lkZjBqsLAraCopl2au6a%2BxrJm