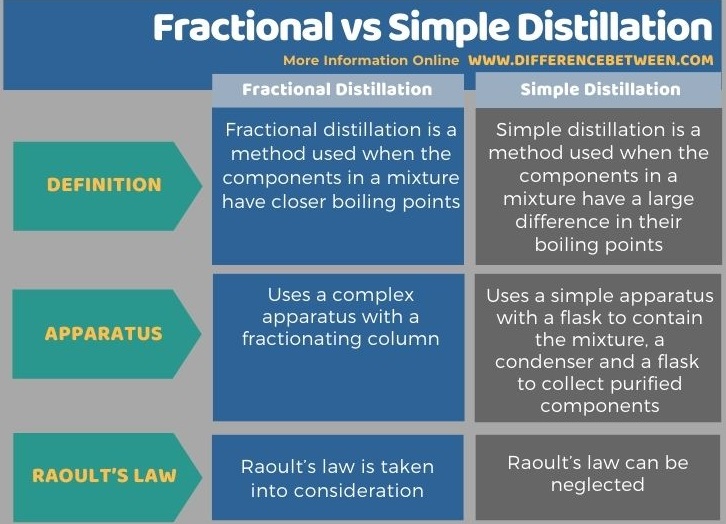
The key difference between fractional and simple distillation is that fractional distillation is used when the components in the mixture have closer boiling points, while simple distillation is used when the components in the mixture have a large difference in their boiling points.
Distillation is a physical separation method that is used to separate compounds from a mixture. It is based on the boiling points of the components in the mixture. When a mixture has different components with different boiling points, they evaporate at different times when heated. This principle is used in the distillation technique. Assume there are two substances A and B in a mixture, and A has a higher boiling point. In that case, when boiling, A will evaporate slower than B; therefore, the vapour will have a higher amount of B than A. Here, the proportion of A and B in the vapour phase is different from the proportion in the liquid mixture. From this, we can reach the conclusion that the most volatile substances will be separated from the original mixture, whereas less volatile substances will remain in the original mixture.
CONTENTS
1. Overview and Key Difference
2. What is Simple Distillation
3. What is Fractional Distillation
4. Side by Side Comparison –Fractional vs Simple Distillation in Tabular Form
5. Summary
What is Simple Distillation?
Simple distillation can be carried out in a laboratory. When preparing an apparatus, a round bottom flask should be connected to a column. End of the column is connected to a condenser and cold water should be circulated in the condenser so that when vapour travels through the condenser it gets cooled. Water should travel in the opposite direction of the vapour, and which allows maximum efficiency. The end opening of the condenser is connected to a flask. The entire equipment should be air sealed so that the vapour won’t escape during the process. A heater can be used to supply the heat to the round bottom flask, which contains the mixture to be separated. When heating, the vapour moves up the column and goes into the condenser. When it travels inside the condenser, it becomes cool and liquefies. This liquid is collected in the flask kept at the end of the condenser.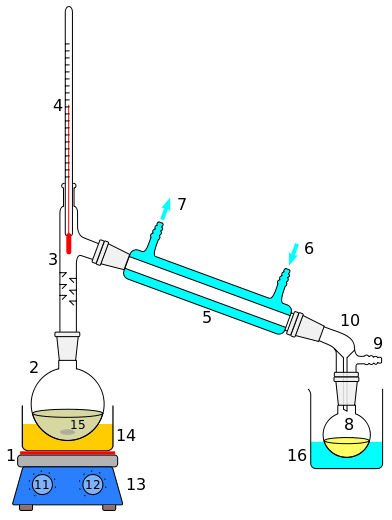
In this method, the vapour produced directly goes into the condenser without travelling through a tall column. Therefore, condensation happens very fast. However, this method may have the disadvantage of producing a distillate with less purity. Since all the volatile compounds tend to evaporate when the heat is supplied, the vapour will contain a mixture of volatile compounds. The ratio of compounds in the mixture can be determined according to their ratio in the original mixture. According to Raoult’s law, the mixture’s composition will be identical to the composition of the vapours at the given temperature and pressure.
If we want to get a good separation in the simple distillation method, it is beneficial to use a mixture that has largely varied boiling points. If not, the components in the mixture should be non-volatile (solid) except the one we need to separate. In this case, only the intended component will evaporate and separate out purely.
What is Fractional Distillation?
When the components in the mixture have closer boiling points, we can use fractional distillation method to separate them out. A fractional column is used in this method. At each level of the fractionating column, the temperature will be different, so the components related to that temperature will remain in that section as vapour while others are condensed back to the round bottom flask.
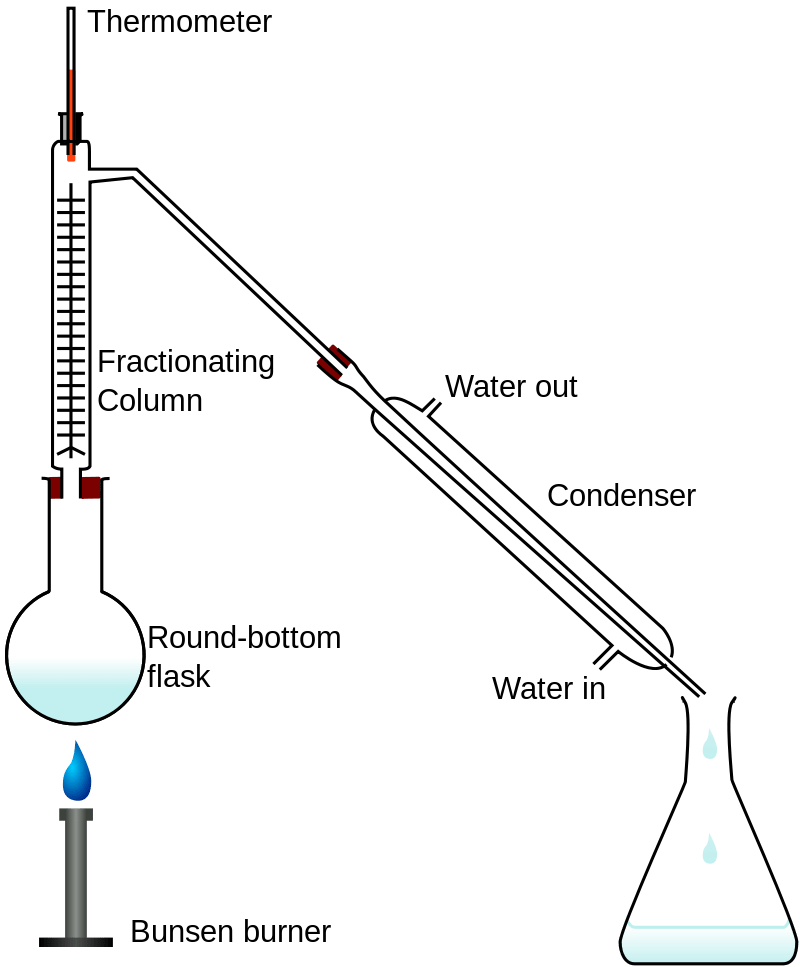
It’s also important to note that the components should be miscible for fractional distillation to be effective. Moreover, fractional distillation is the method used to purify crude oil into various components.
What is the Difference Between Fractional and Simple Distillation?
In fractional distillation method, a fractionating column is used in contrast to simple distillation. When the components in the mixture have closer boiling points, fractional distillation method is used. When they have a large difference in their boiling points, simple distillation can be used. Moreover, Raoult’s law can be neglected in simple distillation but, in fractional distillation, it is taken into consideration.
Summary – Fractional vs Simple Distillation
The key difference between fractional and simple distillation is that fractional distillation method is used when the components in the mixture have closer boiling points, while simple distillation is used when the components in the mixture have a large difference in their boiling points.
Image Courtesy:
1. “Fractional distillation lab apparatus” By Original: Theresa knott (talk · contribs)Derivative work: John Kershaw (talk · contribs) – File:Fractional distillation lab apparatus.png (CC BY-SA 3.0) via Commons Wikimedia
2. “Simple distillation apparatus” By Original PNG by User:Quantockgoblin, SVG adaptation by User:Slashme (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26y0ZqaraGfo66tecCnm2auo2LAqrnPpZxmnJmowaq4y5qroqeeZA%3D%3D