The key difference between equivalence point and endpoint is that the equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the endpoint is the point where the indicator changes its colour.
Titration is a technique we use widely in analytical chemistry to determine acids, bases, oxidants, reductants, metal ions and many other species. In a titration, a chemical reaction takes place. Here, an analyte reacts with a standard reagent, which we call as a titrant. Sometimes we use a primary standard, which is a highly purified and stable solution, as reference material in titrimetric methods. We use an indicator in order to detect the endpoint of the reaction. But, it is not the actual point where the chemical reaction terminates. The actual point is the equivalence point.
CONTENTS
1. Overview and Key Difference
2. What is Endpoint
3. What is Equivalence Point
4. Side by Side Comparison – Equivalence Point vs Endpoint in Tabular Form
5. Summary
What is Endpoint?
In any titration, endpoint is the point where the indicator changes its colour. Or else we can use a change in an instrumental response to identifying the endpoint. For example, HCl and NaOH react 1:1 and produce NaCl and water. For this titration, we can use phenolphthalein indicator, which has a pink colour in the basic medium and turns to colourless in the acidic medium. If we put HCl in the titration flask and to that, if we add a drop of phenolphthalein, it becomes colourless.

Figure 02: Endpoint is the Color Changing Point
During the titration, we can add NaOH from the burette and gradually, HCl and NaOH will react in the flask. If we take the same concentration of the two solutions, when we add an equal amount of NaOH to the flask, the solution in the flask will turn to a light pink colour. This is the point where we stop the titration (endpoint). We consider, at this point, the reaction is completed.
What is Equivalence Point?
The equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample. This is the point where the chemical reaction completes stoichiometrically.
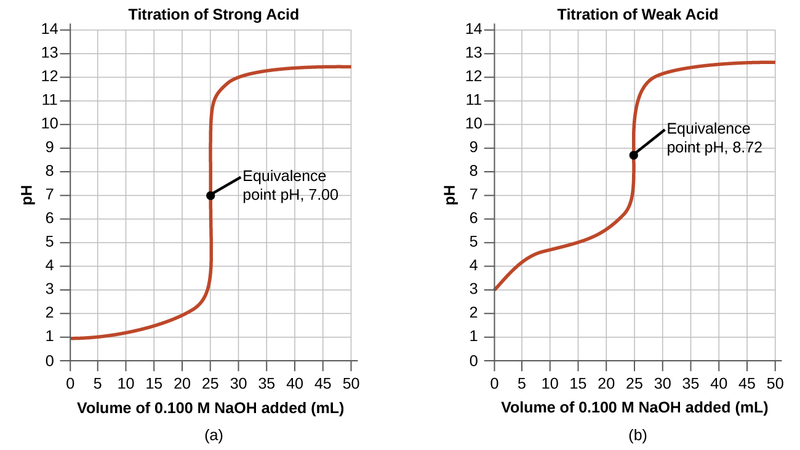
Figure 01: Equivalence Points for Strong Acid and Weak Acid Titration
Although we determine the endpoint from the indicator colour change, it is, most of the time, not the actual endpoint of the reaction. the reaction completes slightly before that point. At this equivalence point, the medium is neutral. In the example discussed in the previous section, after adding an extra NaOH drop, the medium will show the basic colour of phenolphthalein, which we take as the endpoint.
What is the Difference Between Equivalence Point and Endpoint?
Equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the end point is the point where the indicator changes its colour. This is the main difference between equivalence point and endpoint. Moreover, the equivalence point always comes before the endpoint of the titration.
Summary – Equivalence Point vs Endpoint
In any titration, we have two important points; namely, equivalent point and end point of the titration. The key difference between equivalence point and endpoint is that the equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the endpoint is the point where the indicator changes its colour.
Reference:
1. “Equivalence Point.” Wikipedia, Wikimedia Foundation, 15 Apr. 2018. Available here
2. Libretexts. “Titration Fundamentals.” Chemistry LibreTexts, Libretexts, 21 July 2016. Available here
Image Courtesy:
1.”CNX Chem 14 07 titration”By OpenStax (CC BY 4.0) via Commons Wikimedia
2.”Phenolphthalein in Flask”By 384 – Own work, (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26x0K6gr5mcmruksYyppqKmpGKur7CMr6pmnZ6ZvbC1za1m