The key difference between element and compound is that elements can no longer be broken down, while compounds can be broken through chemical reactions.
Both elements and compounds are extremely important in human life; they are present in nature and in man-made developments. Therefore, it’s important to know the difference between element and compound.
CONTENTS
1. Overview and Key Difference
2. What is an Element
3. What is a Compound
4. Side by Side Comparison – Element vs Compound in Tabular Form
5. Summary
What is an Element?
Elements are known to be chemical substances that are quite so simple that they can no longer be chemically broken down into more basic forms. Elements are created out of one kind of atom: an atom, which consists of a nucleus (a cloud consisting of neutrons and protons) encircled by negatively-charged electrons, happens to be the smallest and most basic particle of matter, which explains the basic state of elements.
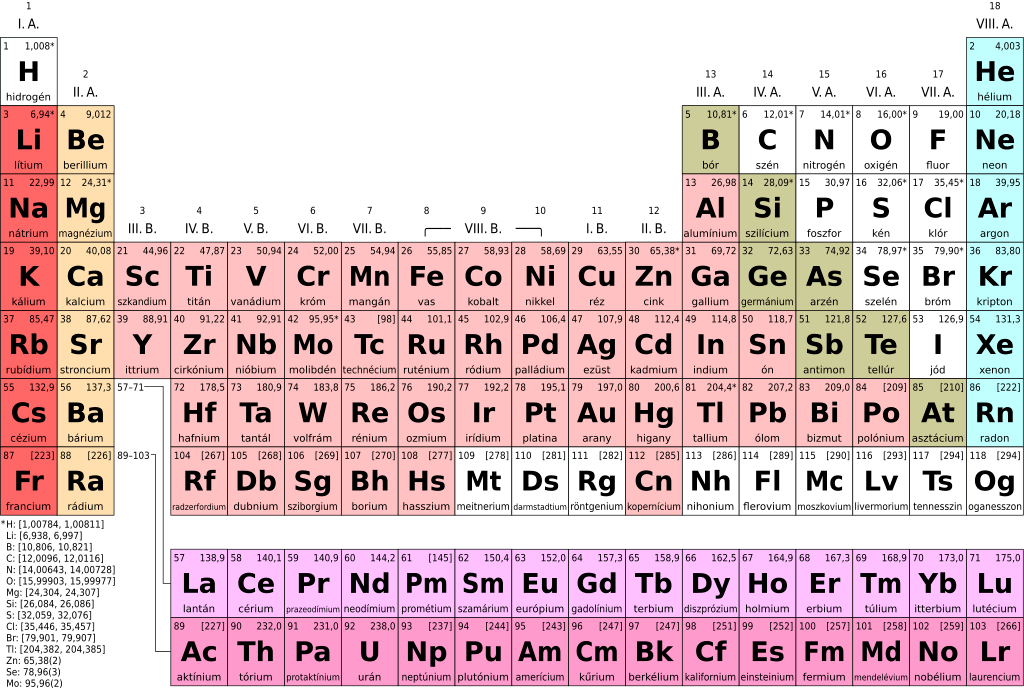
Figure 01: Periodic Table of Elements
Depending on their physical and chemical characteristics, elements are categorized into three divisions: non-metals, metals and metalloids. A chart, called the table of elements, was created by Russian scientist Dmitri Mendeleev, to be able to effectively separate and illustrate the elements according to their types. There are over 118 recognized elements, all of which are symbolized by single or combination of letters. Some of the most popular elements in nature are oxygen and nitrogen.
What is a Compound?
Chemical compounds, on the other hand, are a range of different substances created from combinations of two or more elements which are bonded through chemical processes. The atoms in each element would let go of their individual defining characteristics and are merged to create something altogether different. Ionic bonds form salts, covalent bonds create molecular compounds and metallic bonds make intermetallic compounds. These compounds can take several phases, being solid at most times, but can also turn into liquids and gases depending on how high the temperature applied to them is.
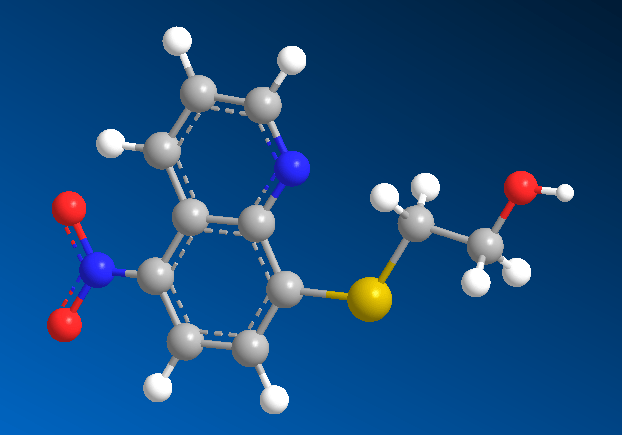
Figure 02: Example of a Compound
In terms of representation, compounds can be defined through different formulas involving numbers and symbols: they are done through the Hill system, wherein Carbon then Hydrogen atoms are classified, followed by all the other elements involved in the compound. Organic compounds are bonded by both, while inorganic compounds do not include carbon and hydrogen. Examples of some of the most widely used chemical compounds are saccharin, an artificial sweetener and sodium chloride, more commonly known as salt.
What is the Difference Between Element and Compound?
Elements are extremely basic and individually consist of one kind of atom. Compounds are elements that are intermingled with one another. Elements can be represented by their symbols while compounds have formulas. Elements can no longer be broken down, while compounds can be broken through chemical reactions. This is the key difference between element and compound.
Moreover, the distinguishing factor for elements is their atomic number, while compounds can be interpreted through their chemical bonds.
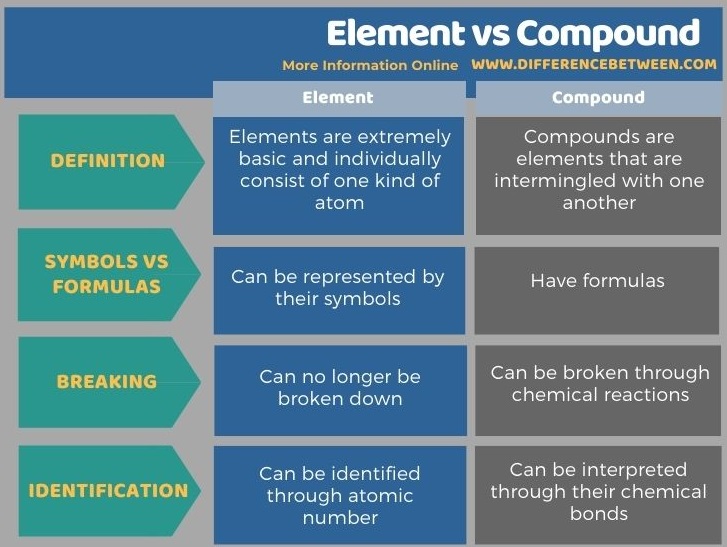
Summary – Element vs Compound
Both elements and compounds are extremely important in human life; they are present in nature and in man-made developments as well such as jewellery, food additives and cleaning substances. The key difference between element and compound is that elements can no longer be broken down, while compounds can be broken through chemical reactions. Although they have different concepts and different ways of working, they are both quite beneficial to humankind.
Image Courtesy:
1. “Periodic table simple hu” By László Németh – Own work (CC0) via Commons Wikimedia
2. “An example of a lead compound” By Fahadum – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26xy56knqakYq6vsIycpqaon6q7pXs%3D