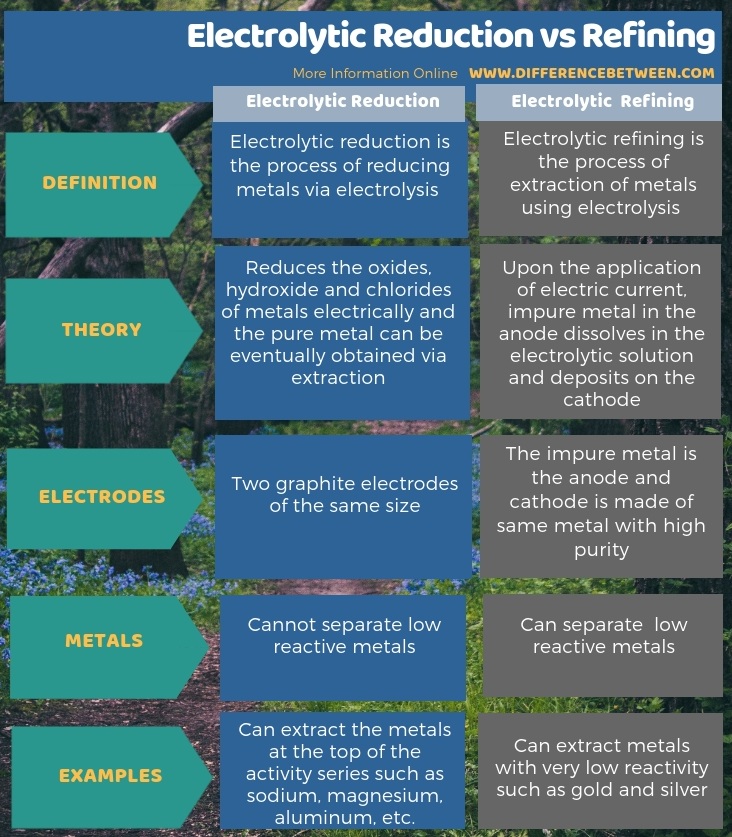
The key difference between electrolytic reduction and refining is that electrolytic reduction method uses graphite electrodes of the same size, whereas electrolytic refining method uses impure metal as the anode and a cathode made of same metal with high purity.
Electrolytic reduction and refining are two important industrial methods we can use to purify a metal. In electrolytic reduction, we can reduce metals into low oxidation states, which enables easy extraction. In electrolytic refining method, the metal from impure anode will deposit on the cathode, allowing us to extract the metal from the cathode.
CONTENTS
1. Overview and Key Difference
2. What is Electrolytic Reduction
3. What is Electrolytic Refining
4. Side by Side Comparison – Electrolytic Reduction vs Refining in Tabular Form
5. Summary
What is Electrolytic Reduction?
The electrolytic reduction is the process of reducing metals via electrolysis. In this process, we use two graphite electrodes of the same size as anode and cathode. The process involves the reduction of oxides, hydroxides and chlorides of metals (which are in a fused state) electrically. Here, we can extract these metals at the cathode. The examples for metals we can obtain through this method include sodium, magnesium, calcium and aluminium. In this method, we can obtain metals with high purity. However, we cannot extract metals with low reactivity using this technique. It is because they form less stable oxides.
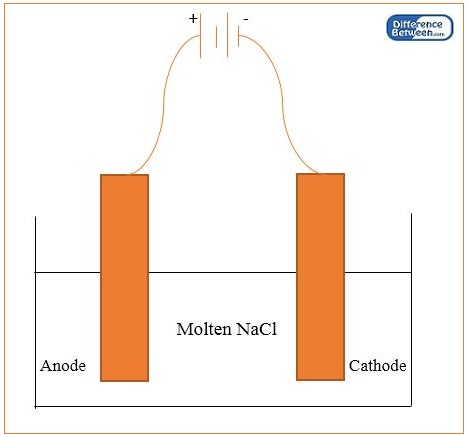
Figure 01: Apparatus for Electrolytic Reduction of Sodium
Usually, most extraction techniques don’t work on metals that are at the top of the activity series. The best method for their extraction is the electrolytic reduction because they are highly electropositive, and we cannot use carbon as a reducing agent to reduce them.
What is Electrolytic Refining?
Electrolytic refining is the process of extraction of metals (metals we can obtain from any refining method) using electrolysis. In this method, the anode is an impure metal block from which we are going to extract the metal while the cathode is a block of the same metal with high purity. Besides, the electrolytic solution is an aqueous solution of the salt of that particular metal (the metal we are going to extract). Then, we can pass an electric current through this electrolytic cell. It will cause the dissolution of the metal from the anode and eventually deposit on the cathode. Therefore, we can collect the pure metal from the cathode. Examples include gold refining, silver refining, copper refining, etc.
What is the Difference Between Electrolytic Reduction and Refining?
The electrolytic reduction is the process of reducing metals via electrolysis, while electrolytic refining is the process of extraction of metals using electrolysis. The key difference between electrolytic reduction and refining is that electrolytic reduction method uses graphite electrodes of the same size, whereas electrolytic refining method uses an impure metal as the anode and a cathode made of same metal with high purity.
Moreover, electrolytic reduction reduces the oxides, hydroxide, and chlorides of metals electrically, and we can obtain pure metal eventually via extraction. However, in electrolytic refining, upon the application of electric current, impure metal in the anode dissolves in the electrolytic solution and deposits on the cathode.
Below infographic shows more information regarding the difference between electrolytic reduction and refining.
Summary – Electrolytic Reduction vs Refining
The electrolytic reduction is the process of reducing metals via electrolysis, while electrolytic refining is the process of extraction of metals using electrolysis. The key difference between electrolytic reduction and refining is that electrolytic reduction method uses graphite electrodes of the same size whereas electrolytic refining method uses the impure metal as the anode and a cathode made of same metal with high purity.
Reference:
1. AZoMining, Written by. “Electrolytic Refining – Mining Fundamentals.” com, 25 Apr. 2014, Available here.
2.“Metals & Non Metals.” Electrolytic Reduction Chemistry, Available here.
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26xy56araqfoca1tcJmqZ6cpZjBqrvNZpinnF2nsqe1zaKloGc%3D