The key difference between electrocyclic and cycloaddition reaction is that electrocyclic reactions are rearrangement reactions, whereas cycloaddition reactions are addition reactions.
Both electrocyclic reactions and cycloaddition reactions are forms of organic chemical reactions that are important in organic synthesis of chemical compounds. They have different mechanisms of action; therefore, we can categorize electrocyclic reactions and cycloaddition reactions into two different groups as rearrangement reactions and addition reactions, respectively.
CONTENTS
1. Overview and Key Difference
2. What is an Electrocyclic Reaction
3. What is a Cycloaddition Reaction
4. Side by Side Comparison – Electrocyclic vs Cycloaddition Reaction in Tabular Form
5. Summary
What is an Electrocyclic Reaction?
An electrocyclic reaction is a type of pericyclic rearrangement in organic chemistry which gives the net result as a conversion of a pi bond into a sigma bond or vice versa. There are different types of electrocyclic reactions because it is a broad branch of organic chemistry. Some categories include photochemical reactions, thermal reactions, ring-opening or ring-closing reactions, etc.
A classic example of an electrocyclic reaction is the thermal ring-opening reaction of the cis-isomer of 3,4-dimethylcyclobutene. This reaction yields cis,trans-hexa-2,4-diene. Similarly, if we are using the trans-isomer of the reactant molecule, then the end result is also a trans diene. The reaction is as follows:
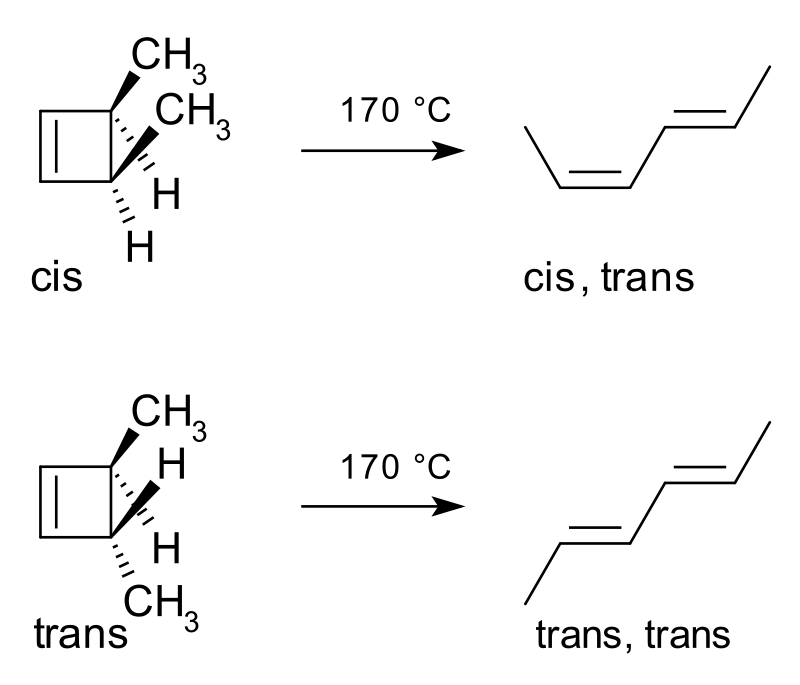
Figure 01: A Classic Example of an Electrocyclic Reaction
The above reaction occurs through the frontier-orbital method. Here, the sigma bond in the reactant opens, forming p orbitals that have the same symmetry as the HOMO of the product, hexadiene. This conversion occurs through a conrotatory ring-opening method that results in opposite signs for the terminal lobes. This conversion of orbitals is shown below.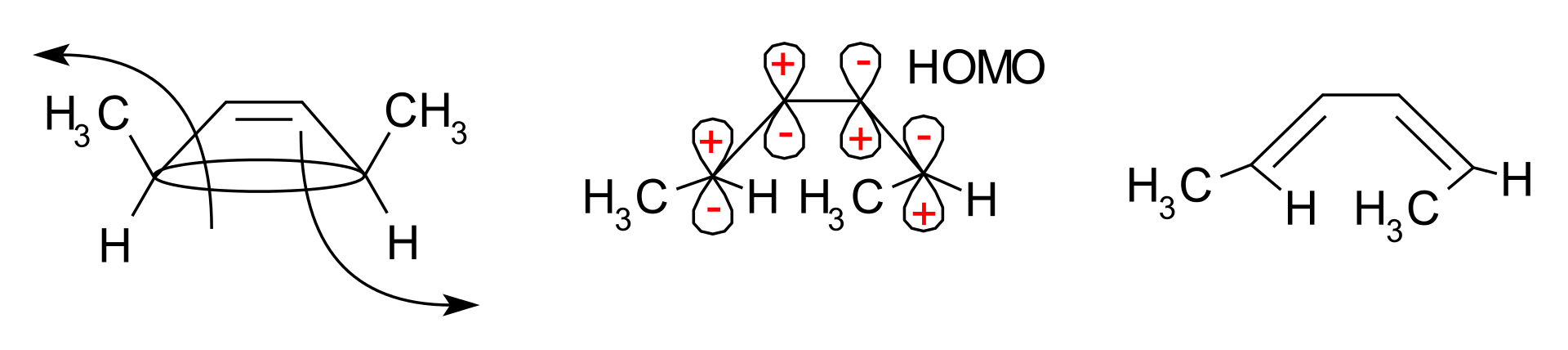
Generally, an electrocyclic reaction shows stereospecificity. That means, we can predict the cis-trans geometry of the final product. As the first step of this prediction, we should determine if the reaction proceeds thorough conrotation or disrotation. After this determination, we can examine the starting molecule to determine if the final product is a cis-isomer or trans-isomer.
What is Cycloaddition Reaction?
Cycloaddition reaction is a type of chemical reaction in organic chemistry where two or more unsaturated molecules combine with each other to form a cyclic adduct. This reaction causes a net reduction of the bond multiplicity. We can name this resulting reaction as a cyclization reaction. Generally, cycloadditions are concerted. Therefore, we can classify them as pericyclic reactions. Similarly, nonconcerted cycloadditions are not pericyclic. Cycloaddition reactions are a type of addition reactions which allow carbon-carbon bond formation without using an electrophile or a nucleophile.
There are different types of cycloadditions, such as thermal cycloaddition, photochemical cycloaddition, Diels-Alder reaction, Huisgen cycloaddition, Cheletropic reactions, etc. Usually, Diels-Alder reactions are the most important cycloaddition reactions.
What is the Difference Between Electrocyclic and Cycloaddition Reaction?
Both electrocyclic reactions and cycloaddition reactions are important in organic synthesis of chemical compounds. The key difference between electrocyclic and cycloaddition reaction is that electrocyclic reactions are rearrangement reactions, whereas cycloaddition reactions are addition reactions.
Moreover, an electrocyclic reaction involves the conversion of a pi bond into a sigma bond or vice versa, while a cycloaddition reaction involves a combination of two or more unsaturated compounds to form a cyclic compound.
The following infographic displays the difference between electrocyclic and cycloaddition reaction in tabular form.
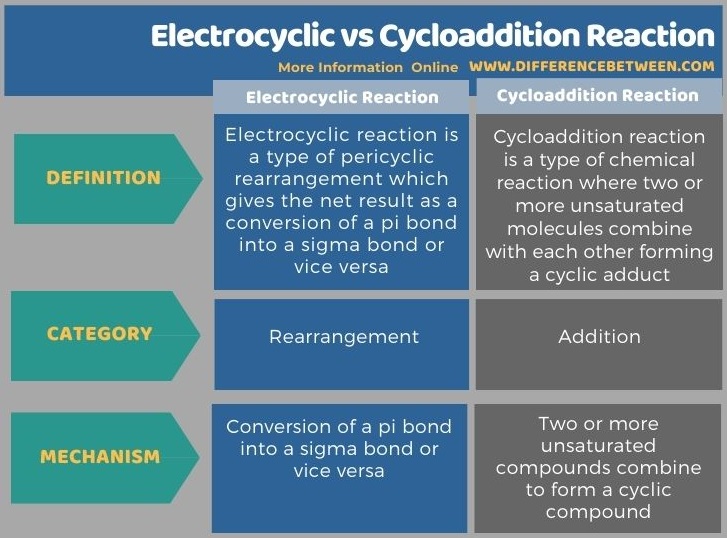
Summary – Electrocyclic vs Cycloaddition Reaction
Both electrocyclic reactions and cycloaddition reactions are important in organic synthesis of chemical compounds. The key difference between electrocyclic and cycloaddition reaction is that electrocyclic reactions are rearrangement reactions whereas cycloaddition reactions are addition reactions.
Reference:
1. Electrocyclic reaction. (2020, August 27). Retrieved September 02, 2020, Available here.
Image Courtesy:
1. “Winterdimethylcyclobutene” By V8rik at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
2. “Dimethylcyclobutene ringopening mechanism” By V8rik at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26xy56araqfmMakuMicZJqmlGKwuq%2FLqJidnJmptrC6jKucmpuknryvew%3D%3D