The key difference between diffusion and effusion is that diffusion occurs when the holes in a barrier are larger than the mean free path of gas but, effusion occurs when the holes in a barrier are smaller than the mean free path of a gas.
Diffusion and effusion are properties of gases and students confuse a lot between these properties because of similar sounding names. Though both diffusion and effusion involve gases, and how gases flow and on what factors this flow rate depends, the two phenomena are different from one another. The manner in which they are different will be discussed in this article.
CONTENTS
1. Overview and Key Difference
2. What is Diffusion
3. What is Effusion
4. Side by Side Comparison – Diffusion vs Effusion in Tabular Form
5. Summary
What is Diffusion?
Diffusion of a gas is the process of expanding the gas to a new volume through a barrier that has holes, which are larger than the mean free path of the gas. The mean free path is the average distance that an individual gas molecule travels before it colloid with another gas molecule.
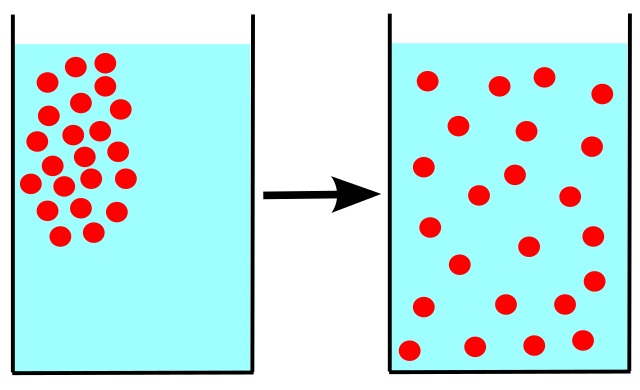
Figure 01: Process of Diffusion
However, if there is no barrier, then we consider a large hole that is large enough to cover the boundary between the gas and the new volume (to which the gas is going to expand). Furthermore, the diffusion is slower compared to effusion. It is because the diffusion is limited by the size and kinetic energy of gas molecules.
What is Effusion?
Effusion is another property of gases which allows gasses to move from areas of high pressure to areas of low pressure through a pinhole. In other words, it is the process of expanding gas through a barrier with one or more small holes; the barrier prevents the distribution of gas unless the gas molecules happen to travel through the holes. Here, the term “small holes” means the holes that have a diameter less than the mean free path of the gas.
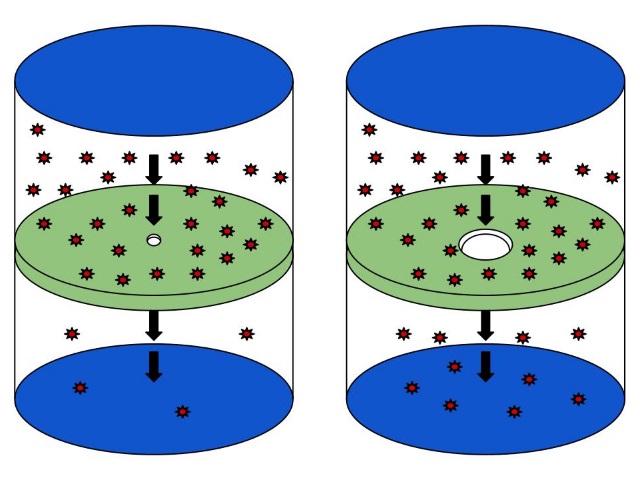
Figure 02: Effusion of a Gas
Typically, the effusion is faster than diffusion because there is no need for gas molecules to move around other gas molecules to find their destination. Specifically, negative pressure on the gas will quicken the process of effusion.
What is the Difference Between Diffusion and Effusion?
Diffusion occurs when the holes in a barrier are larger than the mean free path of gas whereas effusion occurs when the holes in a barrier are smaller than the mean free path of a gas. This is the key difference between diffusion and effusion. Moreover, diffusion of gas molecules through the barrier is easier than the movement of gas molecules via effusion. It is mainly due to the size of the holes in the barrier; barrier has a diameter larger than the mean free path of gas molecules in diffusion whereas barrier has a diameter smaller than the mean free path of gas molecules in effusion. Therefore, this is also a significant difference between diffusion and effusion.
However, the rate of diffusion is slower compared to effusion. It is because the diffusion is limited by the size and kinetic energy of gas molecules. In addition, the gas molecules need to move around other gas molecules to find their destination through the barrier which does not happen in effusion.
The below infographic presents the difference between diffusion and effusion in tabular form.
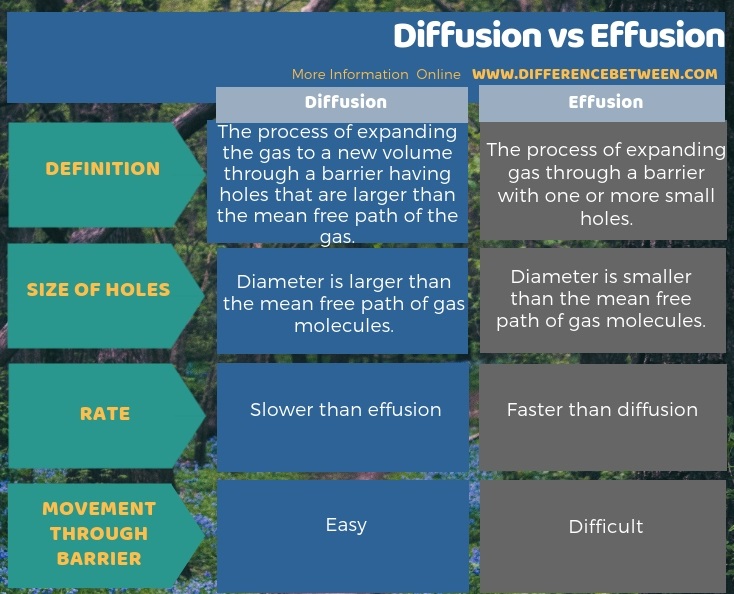
Summary – Diffusion vs Effusion
In this article, we discussed the terms diffusion and effusion regarding gases. The key difference between diffusion and effusion is that diffusion occurs when the holes in a barrier are larger than the mean free path of gas whereas effusion occurs when the holes in a barrier are smaller than the mean free path of a gas.
Reference:
1. Helmenstine, Todd. “What Is The Difference Between Diffusion and Effusion?” ThoughtCo, Sep. 28, 2018. Available here
Image Courtesy:
1.”Diffusion”By JrPol – Own work, (CC BY 3.0) via Commons Wikimedia
2.”Effusion”By Astrang13 – Own work, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26wyJ%2BdrquZpLturc2dZK%2BrXZqzp8HSoqanZw%3D%3D