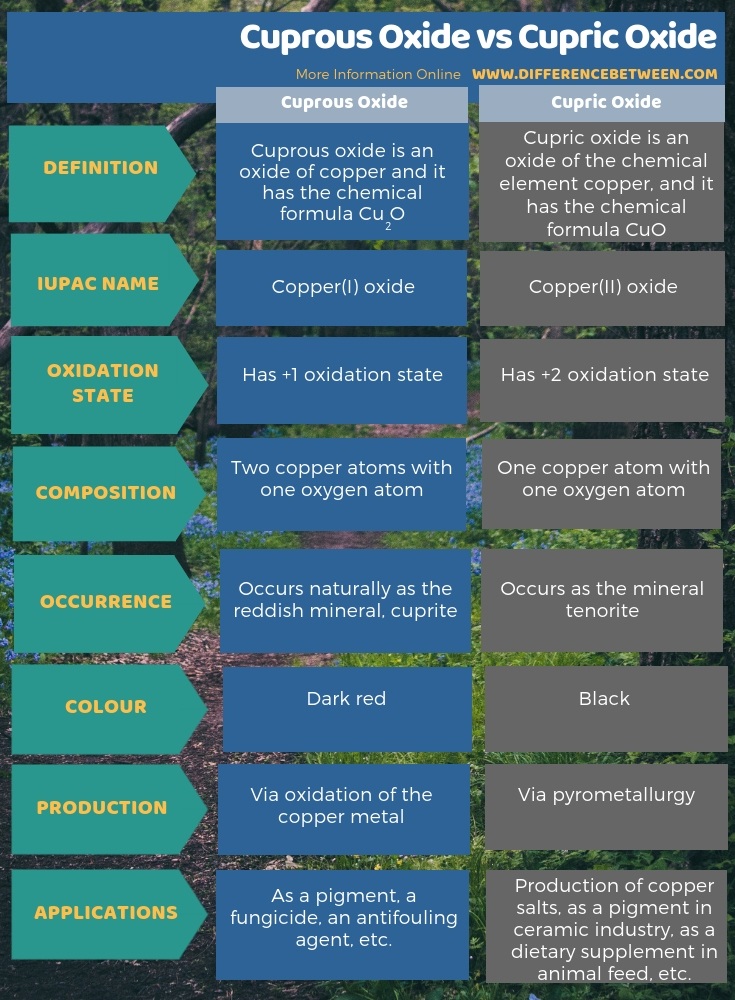
The key difference between cuprous oxide and cupric oxide is that cuprous oxide has a dark red colour whereas cupric oxide has a black colour.
Both cuprous oxide and cupric oxide are compounds of the chemical element copper. These oxides contain different oxidation states of copper. Moreover, in cuprous oxide, there is a +1 oxidation state, and in cupric oxide, there is a +2 oxidation state.
CONTENTS
1. Overview and Key Difference
2. What is Cuprous Oxide
3. What is Cupric Oxide
4. Side by Side Comparison – Cuprous Oxide vs Cupric Oxide in Tabular Form
5. Summary
What is Cuprous Oxide?
Cuprous oxide is an oxide of the chemical element copper, which has the +1 oxidation state of copper. Therefore the IUPAC name of cuprous oxide is copper(I) oxide. It is an inorganic compound and has the chemical formula Cu2O. Additionally, if we look at its structure, two copper atoms are in association with one oxygen atom. Besides, this compound has a red colour. Naturally, we can find it as the reddish mineral, cuprite.

Figure 01: Cuprous Oxide
Furthermore, the most common way to produce this compound is via oxidation of the copper metal.
4 Cu + O2 → 2 Cu2O
Moreover, it forms on silver-plated copper parts if they are exposed to moisture after damaging the silver layer. We call it corrosion or red plague.
When considering properties, cuprous oxide exists as a solid, and it is diamagnetic. It can dissolve in concentrated solutions of ammonia and form a complex; [CuNH3)2]+. Besides, this complex easily oxidizes and forms a blue coloured complex, which is [Cu(NH3)4(H2O)2]2+.
What is Cupric Oxide?
Cupric oxide is an oxide of the chemical element copper, and it has the chemical formula CuO. Here, one copper atom associates one oxygen atom. Copper(II) oxide is its IUPAC name. It occurs as a black solid and is very stable. Besides, this compound naturally occurs as the mineral tenorite. Also, it is a precursor for many copper containing compounds.

Figure 02: Cupric Oxide
Furthermore, we can produce this compound via pyrometallurgy on a large scale. It occurs in a monoclinic crystal system. Here, the copper atom associates with four oxygen atoms in a square planar configuration. Notably, it is a p-type semiconductor.
What is the Difference Between Cuprous Oxide and Cupric Oxide?
Cuprous oxide is Cu2O while cupric oxide is CuO. The key difference between cuprous oxide and cupric oxide is that cuprous oxide has a dark red colour whereas cupric oxide has a black colour. The IUPAC name of cuprous oxide is copper(I) oxide while the IUPAC name of cupric oxide is copper(II) oxide.
Moreover, in cuprous oxide, there is a +1 oxidation state while in cupric oxide, there is a +2 oxidation state. A further difference between cuprous oxide and cupric oxide is that cuprous oxide occurs naturally as the reddish mineral, cuprite where cupric oxide occurs as the mineral tenorite.
The below infographic shows more comparisons related to the difference between cuprous oxide and cupric oxide.
Summary – Cuprous Oxide vs Cupric Oxide
In brief, cuprous oxide and cuprous oxide are oxide compounds of copper metal. The key difference between cuprous oxide and cupric oxide is that cuprous oxide has a dark red colour whereas cupric oxide has a back colour.
Reference:
1. “Copper(I) Oxide.” Wikipedia, Wikimedia Foundation, 17 May 2019, Available here.
Image Courtesy:
1. “CopperIIoxide” By User Walkerma on en.wikipedia (Public Domain) via Commons Wikimedia
2. “CopperIoxide” (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26v1KmpqK2jYry5tcOeZJqmlGKwtrzRoppmp6iesaZ7