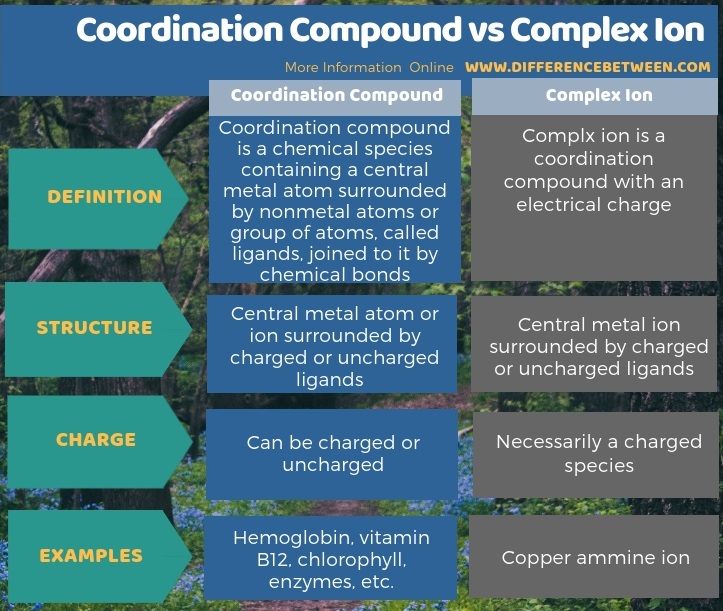
The key difference between coordination compound and complex ion is that coordination compound can be charged or uncharged, whereas complex ion is a charged species.
The terms coordination compound and complex ion come under the coordination chemistry in the branch of inorganic chemistry. Although we often use these terms interchangeably, there is a difference between coordination compound and complex ion.
CONTENTS
1. Overview and Key Difference
2. What is Coordination Compound
3. What is Complex Ion
4. Side by Side Comparison – Coordination Compound vs Complex Ion in Tabular Form
5. Summary
What is Coordination Compound?
Coordination compound is a chemical species containing a central metal atom surrounded by nonmetal atoms or group of atoms called ligands, joined to it by chemical bonds. We name them as coordination compounds because there are coordinate covalent bonds between metal atoms and ligands.
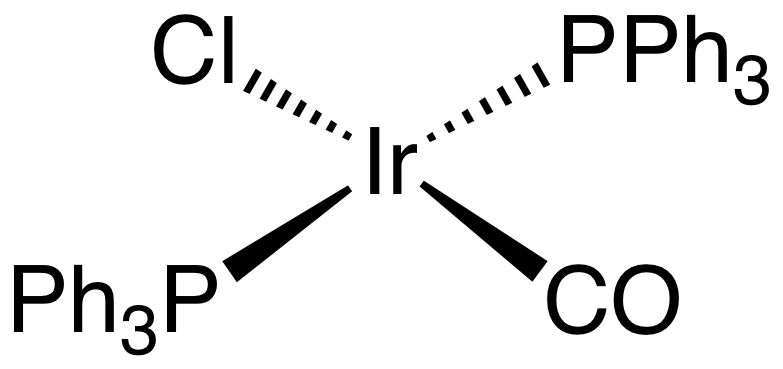
Figure 01: An Uncharged Coordination Compound
These compounds can be either charged or uncharged species. If it is a charged species or ion, then we name it as a complex ion. Some examples of coordination compounds are as follows:
What is Complex Ion?
Complex ion is a coordination compound with an electrical charge. Therefore, this chemical species also contains a central metal atom and ligands bound to it.
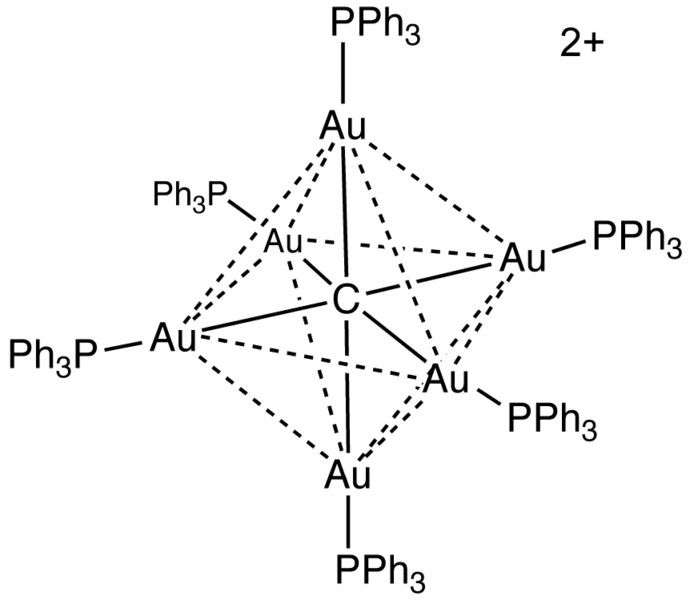
Figure 02: Structure of a Complex Ion
However, the central metal atom is a charged species, so we call it a metal ion. It is the coordination centre and the ligands around it can be charged or uncharged species. Moreover, copper ammine ion is a complex ion.
What is the Difference Between Coordination Compound and Complex Ion?
The key difference between coordination compound and complex ion is that coordination compound can be charged or uncharged, whereas complex ion is a charged species. Moreover, some examples of coordination compounds include haemoglobin, vitamin B12, chlorophyll, enzymes, etc. while copper ammine ion is a good example for complex ions.
Summary – Coordination Compound vs Complex Ion
In summary, coordination compound and complex ion are two different terms. The key difference between coordination compound and complex ion is that coordination compound can be charged or uncharged whereas complex ion is a charged species.
Reference:
1. Helmenstine, Anne Marie, “Coordination Compound Definition.” ThoughtCo, Jul. 11, 2019, Available here.
Image Courtesy:
1. “Vaskas” By Smokefoot – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Au6C(PPh3)6” By Smokefoot – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vzqipnaGelsGqu81mmqiloKTCr7CMmqWdZZOkurG4xLFkoqeeZA%3D%3D