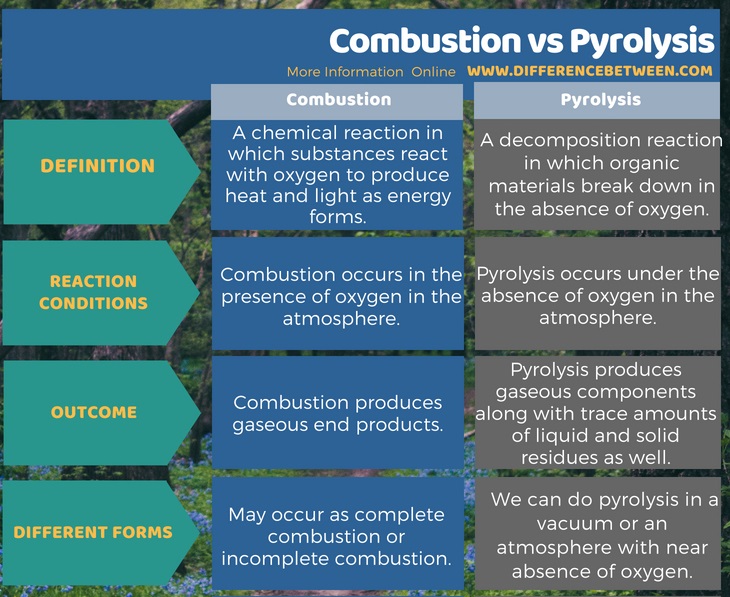
The key difference between combustion and pyrolysis is that the combustion occurs in the presence of oxygen whereas the pyrolysis occurs in the absence (or near absence) of oxygen.
Both combustion and pyrolysis are thermochemical reactions. Combustion is exothermic because it produces heat and light energy. Pyrolysis, on the other hand, is a decomposition reaction in which organic matter decomposes when we provide heat energy. Although both are thermochemical reactions, there is some difference between combustion and pyrolysis.
CONTENTS
1. Overview and Key Difference
2. What is Combustion
3. What is Pyrolysis
4. Side by Side Comparison – Combustion vs Pyrolysis in Tabular Form
5. Summary
What is Combustion?
Combustion is a chemical reaction in which substances react with oxygen to produce heat and light as energy forms. In common, we call it “burning”. The light energy that comes out as an outcome of this reaction appears as a flame. However, most of the energy released as heat. There are two types of complete and incomplete combustion.
Complete Combustion
This type of reactions occurs in the presence of excess oxygen. It gives a limited number of products as the outcome; for example, if we burn fuel, it gives carbon dioxide and water. If we burn a chemical element, it gives the most stable oxide of that element.
Incomplete Combustion
This type of reaction occurs in the presence of a less amount of oxygen. Unlike complete combustion, this gives a high number of products as the outcome; for example, if we burn a fuel in the presence of a less amount of oxygen, it gives carbon monoxide, carbon dioxide and water. Sometimes, it gives unburnt carbon as well.

Figure 01: Combustion means Burning
Among the uses of combustion reactions, the most important one is the production of energy via burning fuels. Ex: For automobiles, industries, etc. In addition to that, we can produce fire from these reactions. Ex: for cooking. Moreover, we can use these reactions in order to identify the chemical elements according to their flame color.
What is Pyrolysis?
Pyrolysis is a decomposition reaction in which organic materials break down in the absence of oxygen. We need to apply heat for this reaction to progress. Hence, we can increase the rate of reaction via increasing the amount of heat provided. Typically, this reaction takes place at or above 430oC. However, most of the times, we do these reactions in near absence of oxygen because it is very difficult to obtain an atmosphere free of oxygen. The end product of this reaction can be in the gas phase, liquid phase or the solid phase. Most of the times, it produces gases. If it produces a liquid, we call this liquid “tar”. If it is a solid, typically, can be charcoal or biochar.
In most cases, pyrolysis converts organic matter into their gaseous components, a solid residue of carbon and ash, and a liquid called pyrolytic oil. Here, we use two major methods to remove any contaminants from a substance; destruction and removal. Destruction process breaks down the contaminants into small compounds while removal process separates the contaminants from the desired substance.
The uses of this reaction are in industries in order to produce charcoal, activated carbon, methanol, etc. Moreover, it can destroy semi-volatile organic compounds, fuels, etc. in addition to that; we can use this process to treat organic waste coming out from factories.
What is the Difference Between Combustion and Pyrolysis?
Combustion is a chemical reaction in which substances react with oxygen to produce heat and light as energy forms. It occurs in the presence of oxygen in the atmosphere. More importantly, it produces gaseous end products. Pyrolysis is a decomposition reaction in which organic materials break down in the absence of oxygen. It occurs under the absence of oxygen in the atmosphere. Unlike combustion, it produces gaseous components along with trace amounts of liquid and solid residues as well.
Summary – Combustion vs Pyrolysis
Both combustion and pyrolysis are thermochemical reactions. But, there are differences between combustion and pyrolysis. The key difference between combustion and pyrolysis is that combustion occurs in the presence of oxygen whereas pyrolysis occurs in the absence (or near absence) of oxygen.
Reference:
1. “Combustion.” Wikipedia, Wikimedia Foundation, 29 July 2018. Available here
2. “Pyrolysis.” Wikipedia, Wikimedia Foundation, 27 July 2018. Available here
Image Courtesy:
1.”Burning Fire” by Petr Kratochvil (CC0) via PublicDomainPictures.net
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vzqaZrquknryvecCnm2aoqae8rcXSoqpo