The key difference between chemisorption and physisorption is that chemisorption is a type of adsorption in which the adsorbed substance is held by chemical bonds whereas physisorption is a type of adsorption in which the adsorbed substance is held by intermolecular forces.
Chemisorption and physisorption are generally important chemical concepts we can use to describe the adsorption mechanism of a substance on to a surface. Chemisorption is the adsorption by chemical means while physisorption is the adsorption by physical means.
CONTENTS
1. Overview and Key Difference
2. What is Chemisorption
3. What is Physisorption
4. Side by Side Comparison – Chemisorption vs Physisorption in Tabular Form
5. Summary
What is Chemisorption?
Chemisorption is the process in which the adsorption of a substance on to a surface is driven by chemical means. Here, the adsorbate attaches with the surface via chemical bonds. Therefore, this mechanism involves a chemical reaction between adsorbate and the surface. Here, chemical bonds may break down and form at the same time. Moreover, the chemical species that build up the adsorbate and surface undergo changes due to this bond breaking and formation.
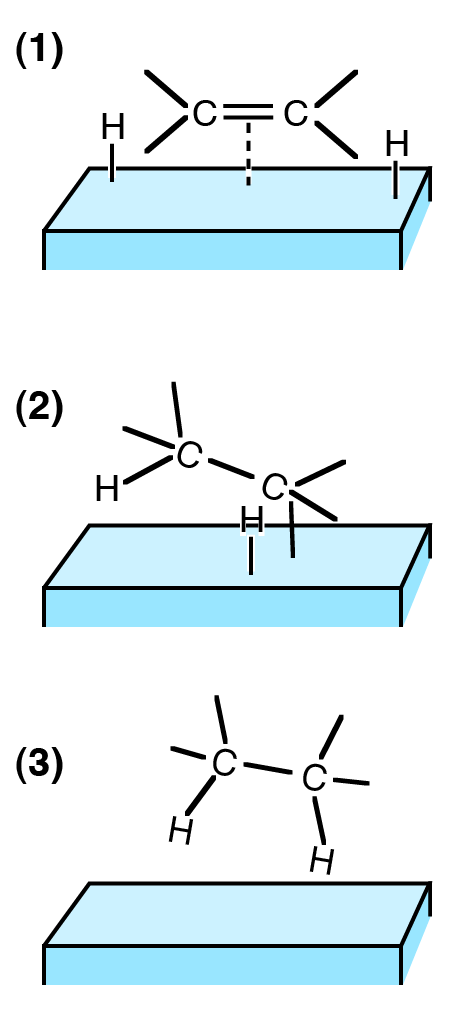
Figure 01: Three Steps for Chemisorption
A common example is corrosion, which is a macroscopic phenomenon we can observe from the naked eye. Furthermore, the types of bonds that can form between adsorbate and surface include covalent bonds, ionic bonds and hydrogen bonds.
What is Physisorption?
Physisorption is the process in which the adsorption of a substance on to a surface is driven by physical means. That means; there are no chemical bond formations, and this process involves intermolecular interactions such as Van der Waal forces. The adsorbate and surface exist intact. Therefore, there is no involvement of the electronic structure of atoms or molecules.
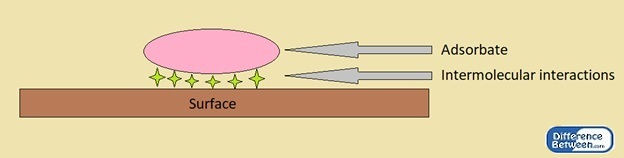
A common example is the Van der Waals forces between surfaces and foot hair of geckos, which help them to climb vertical surfaces.
What is the Difference Between Chemisorption and Physisorption?
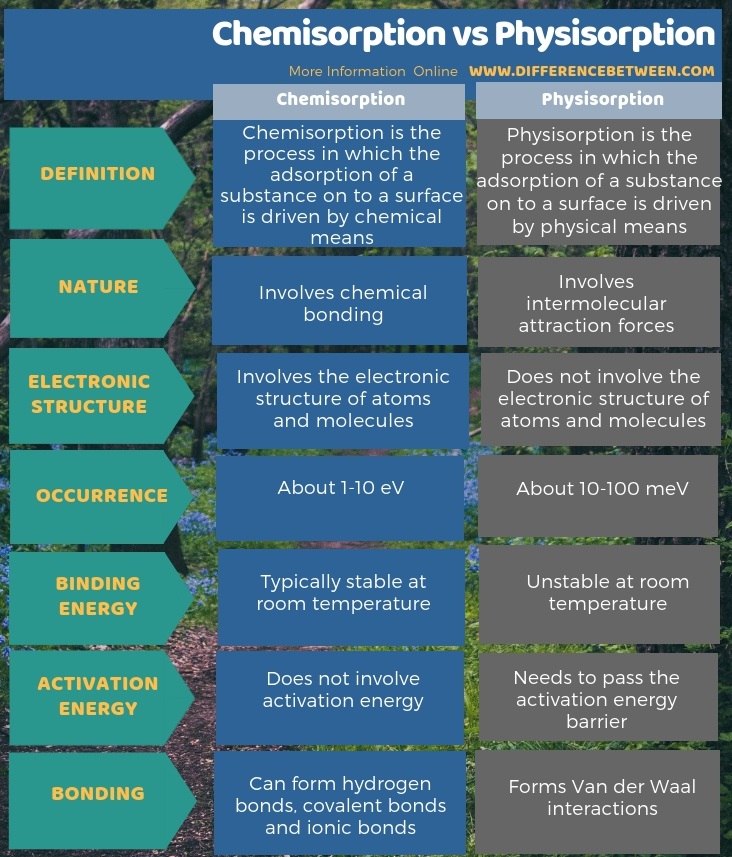
The key difference between chemisorption and physisorption is that in chemisorption, chemical bonds hold the adsorbed substance whereas, in physisorption, intermolecular forces hold the adsorbed substance. Moreover, chemisorption can form hydrogen bonds, covalent bonds and ionic bonds but physisorption forms Van der Waal interactions only. So, we can consider this also as a difference between chemisorption and physisorption. The binding energy for chemisorption ranges from 1-10 eV while in physisorption it is about 10-100 meV.
Below infographic shows more comparisons regarding the difference between chemisorption and physisorption.
Summary – Chemisorption vs Physisorption
The key difference between chemisorption and physisorption is that chemisorption is a type of adsorption in which chemical bonds hold the adsorbed substance, whereas physisorption is a type of adsorption in which intermolecular forces hold the adsorbed substance.
Reference:
1. Murr, L.e. “Imaging Systems and Materials Characterization.” Materials Characterization, vol. 60, no. 5, 2009, pp. 397–414., doi:10.1016/j.matchar.2008.10.013.
Image Courtesy:
1. “Hydrogenation on catalyst” By Michael Schmid – Drawing created myself (CC BY 1.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vx56koqufp721tc6nZJqmlGK9qcXSoqqoqqCptrC6jg%3D%3D