The key difference between chelate and macrocyclic ligands is that a chelate is a compound containing a central metal atom bonded to a ligand having at least two or more donor sites whereas a macrocyclic ligand is a large cyclic structure having three or more donor sites.
A ligand is a molecule or an ion that can bind to a metal atom or ion via donating its lone electron pairs to form coordinate covalent bonds. The donor sites are the sites at which the ligands donate lone electron pairs to the metal atom or ion.
CONTENTS
1. Overview and Key Difference
2. What are Chelates
3. What are Macrocyclic Ligands
4. Side by Side Comparison – Chelate vs Macrocyclic Ligands in Tabular Form
5. Summary
What are Chelates?
A chelate is a compound containing a central metal atom bonded to a ligand having at least two or more donor sites. Therefore, chelate is the whole complex that contains the central metal atom and the ligand. This complex is also known as coordination complex or coordination compound. Some coordination compounds have two or more ligands bonded to the central metal atom, but a chelate has only one ligand.
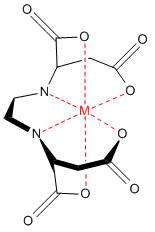
Figure 01: EDDS metal complex
There are several categories of ligands. Their name indicates how many coordinate covalent bonds they can form. For example, if a ligand can form only one coordinate covalent bond per molecule, it is known as a monodentate ligand. Likewise, if there are two donor sites, then it is a bidentate ligand. The denticity of ligands describes this categorization. Since the ligand is attached to the central metal atom via two or more donor sites in a chelate, the ligand is either a bidentate or a polydentate ligand. Most of the times, the ligand of a chelate is a cyclic or a ring structure. These ligands are also known as chelating agents.
What are Macrocyclic Ligands?
A macrocyclic ligand is a large cyclic structure having three or more donor sites. A macrocyclic ligand is essentially a large cyclic structure. There are at least three or more donor sites in a macrocyclic ligand. These ligands show a very high affinity for metal ions.
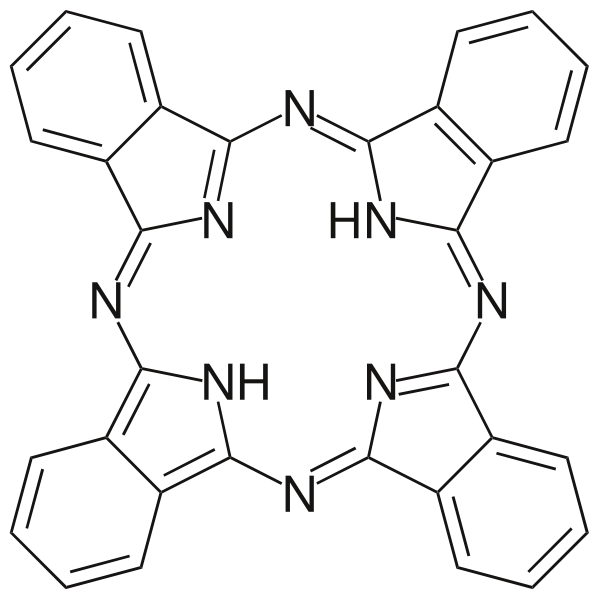
Figure 1: Phthalocyanine is an important example for macrocyclic ligands.
The macrocyclic ligands are essentially polydentate. Therefore, these ligands provide less conformational freedom for the metal ion. That is, these ligands are “pre-organized” for binding. When a macrocyclic ligand bonds to a metal ion, the whole structure is known as a macrocyclic complex. One of the most common macrocyclic ligands is phthalocyanine. The common use of these ligands are dyes and pigments.
What is the Difference Between Chelate and Macrocyclic Ligands?
Chelate vs Macrocyclic Ligands | |
| A chelate is a compound containing a central metal atom bonded to a ligand having at least two or more donor sites. | A macrocyclic ligand is a large cyclic structure having three or more donor sites. |
| Nature | |
| A coordination compound | A donor molecule |
| Donor Site | |
| The ligand has at least two or more donor sites. | The ligand has at least three or more donor sites. |
| Denticity | |
| The ligand is either bidentate or polydentate. | Essentially a polydentate ligand. |
Summary – Chelate vs Macrocyclic Ligands
Chelates are coordination compounds. Macrocyclic ligands are donor molecules which can donate lone electron pairs to form coordinate covalent bonds with a central metal atom or ion. The key difference between chelate and macrocyclic ligands is that a chelate is a compound containing a central metal atom bonded with a ligand having at least two or more donor sites whereas a macrocyclic ligand is a large cyclic structure having three or more donor sites.
Image Courtesy:
1. “EDDS metal complex” By Yidele (talk) – Own work – using ChemDraw Ultra 11.0 (Public Domain) via Commons Wikimedia
2. “Phthalocyanine” By Choij – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vx56jmqyVYq6vsIymmJyqn5jGpLjInGSloZeWu6W%2Fjg%3D%3D