The key difference between cellobiose and cellulose is that cellobiose is a disaccharide, whereas cellulose is a polysaccharide.
Cellobiose and cellulose are carbohydrate compounds. We can categorize carbohydrates into different categories such as monosaccharides, disaccharide and polysaccharides, depending on the structure and the complexity of the carbohydrate. A monosaccharide is a simple sugar, while a disaccharide is a combination of two monosaccharides and a polysaccharide is a combination of many monosaccharide units.
CONTENTS
1. Overview and Key Difference
2. What is Cellobiose
3. What is Cellulose
4. Side by Side Comparison – Cellobiose vs Cellulose in Tabular Form
5. Summary
What is Cellobiose?
Cellobiose is a carbohydrate with the chemical formula C12H22O11. We can categorize it as a disaccharide. It is a reducing sugar. That means; the cellobiose can act as a reducing agent because it has a free ketone group in its structure. Cellobiose has two beta-glucose molecules linked via beta 1-4 glycosidic linkage. However, it is different from maltose because the configuration at the glycosidic bond is different. We can hydrolyze this compound into glucose by enzymatic means or by chemical means using an acid.
When considering the structure of cellobiose, there are eight free alcohol groups along with an acetal group and a hemiacetal group. These groups provide the molecule with an ability to form strong intermolecular hydrogen bonds.
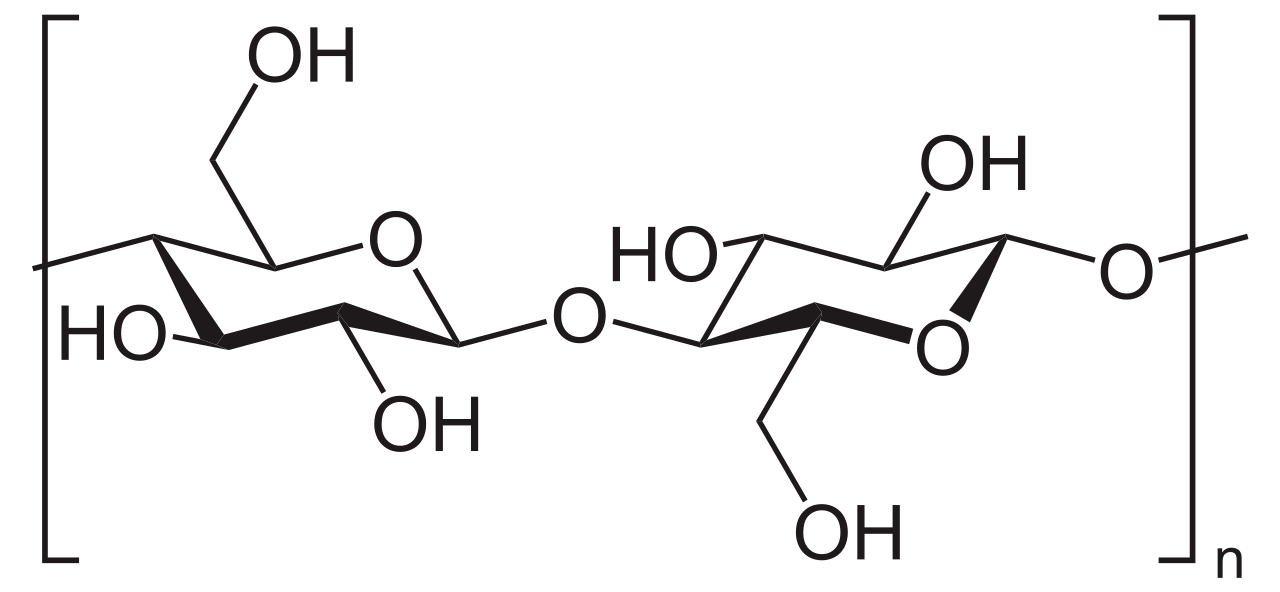
Figure 01: Chemical Structure of Cellobiose
We can obtain cellobiose from cellulose or cellulose-containing materials such as paper, cotton, etc. Here, we need enzymatic or acidic hydrolysis of these materials to obtain cellobiose from these materials. This compound is also important in detecting Crohn’s disease, as an indicator for carbohydrates.
What is Cellulose?
Cellulose is a carbohydrate we can categorize as a polysaccharide and it has the chemical formula (C6H10O5)n. It may contain hundreds to thousands of D-glucose units, linked to each other via beta 1-4 glycosidic bonds. If we produce or isolate it from a source, it appears as a white powder. Cellulose is present as an important structural unit of cell walls in plants and algae. Sometimes, some bacteria species also secrete cellulose to form biofilms. Therefore, we can say that cellulose is the most abundant polymer material on Earth.
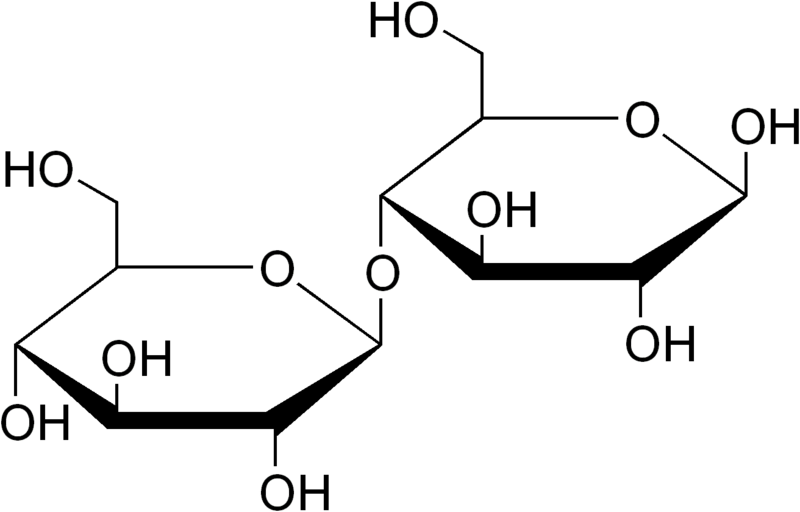
Figure 02: Chemical Structure of Cellulose
Cellulose is an odourless and colourless compound. It is insoluble in water and many organic solvents. Furthermore, it is a chiral compound, and is biodegradable as well. We can break down cellulose into glucose units by adding highly concentrated mineral acids at a higher temperature. Compared to starch, this compound is highly crystalline, and it can also undergo conversion from crystalline to an amorphous structure upon heating.
When considering the applications of cellulose, it is mainly used in the production of paper and paperboard. We can also use it for the production of cellophane and rayon. In addition to these, there are research studies exploring the conversion of cellulose into biofuel.
What is the Difference Between Cellobiose and Cellulose?
The terms cellobiose and cellulose mainly fall under the field of biochemistry. These are carbohydrate compounds. The key difference between cellobiose and cellulose is that the cellobiose is a disaccharide, whereas the cellulose is s polysaccharide. Moreover, cellobiose is a reducing sugar while cellulose is a non-reducing sugar.
Below infographic tabulates the difference between cellobiose and cellulose.
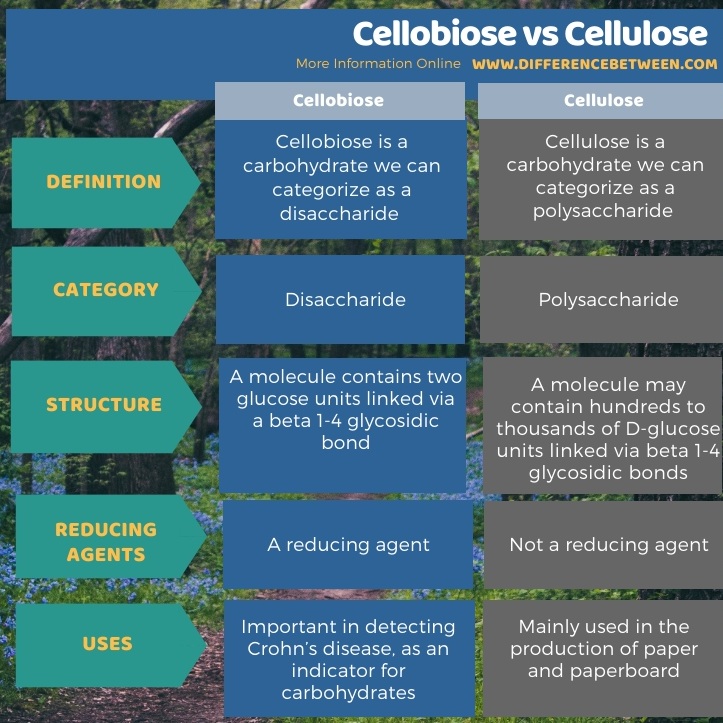
Summary – Cellobiose vs Cellulose
Cellobiose and cellulose are carbohydrate compounds. The key difference between cellobiose and cellulose is that cellobiose is a disaccharide, whereas cellulose is s polysaccharide. Moreover, cellobiose is a reducing sugar while cellulose is a non-reducing sugar.
Reference:
1. Zhang, Guoqiang, and Qinhong Wang. “Functional Carbohydrates.” Functional Carbohydrates, Feb. 2017, pp. 269–298., doi:10.1201/9781315371061-9.
2. “Cellobiose.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, Available here.
3. “Cellulose.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 7 Feb. 2019, Available here.
Image Courtesy:
1. “Cellobiose” (CC BY-SA 3.0) via Commons Wikimedia
2. “Cellulose Sessel” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vxKWjqJqZpMCmecCnm2ablaG5trjOrJxoW2qzh7Wx1610fJ2cobyjtc6snF5qYKvAZn6PfJylpKWhvLSxjGV6nqScpK%2Bqu9KeXGtokaOxZn6PnJylpKWhvLSxhGtnmqqVWn9xr8CrmaigqZm%2FosDEXmlpm5%2BivbDBzZ2qZ2SZqHJzfMBeaWmmn6Nyc5DRnpuum5mjtGZ%2Bj6ysoJmiYw%3D%3D