The key difference between cationic and anionic dyes is that cationic dyes are basic, whereas anionic dyes are acidic.
Dyes are natural or synthetic substances we can use to add colour or change the colour of an object. There are different forms of dyes, such as cationic and anionic dyes.
CONTENTS
1. Overview and Key Difference
2. What are Cationic Dyes
3. What are Anionic Dyes
4. Cationic vs Anionic Dyes in Tabular Form
5. Summary – Cationic vs Anionic Dyes
What are Cationic Dyes?
Cationic dyes are dye materials having components that make them dissociate into positively charged ions in an aqueous solution. In other words, cationic dyes separate into ions and form cations when added to water. Moreover, when these cationic dyes are added to fibres, the cations can interact with the negatively charged groups on fibre molecules, forming salts. These salts can further be firmly attached to the fibres. Therefore, it can stain the fibre.
Typically, cationic dyes are made based on alkaline dyes. Therefore, the principle of the combination of cationic dyes with fibres is through the combination of cations with the acidic groups present in the fibre. Originally, this type of dyes was useful in staining silk, leather, paper, and cotton. In addition, these dyes have applications in the production of ink and in copying papers. Moreover, the demand for this dye in the textile industry has increased due to the introduction of synthetic fibres.
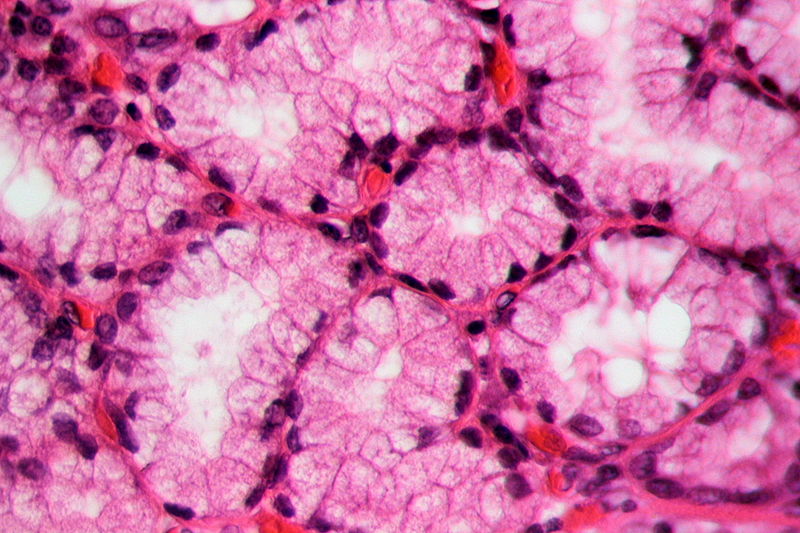
Figure 01: Lee’s Stain used in Gallbladder Cells
Let us consider the dyeing of synthetic fibres with cationic dyes. First, the cationic dye gets absorbed by the surface of the fibre, and it diffuses into the interior of the fibre at high temperatures. There, the dye binds to the active acid groups of the fibre. However, the number of dye molecules that can bind with the acid groups is limited, and this number can be increased by adjusting the temperature and fibre composition. We can characterize the dyeing ability of cationic dyes using affinity and diffusibility.
What are Anionic Dyes
Anionic dyes are dyes having components that can make the dye molecule dissociate into negatively charged ions in an aqueous solution. In other words, anionic dyes separate into ions and form anions when added to water. Usually, anionic dyes are acidic dyes.

Figure 02: The Chemical Structure of Acid Red 88 Dye
This type of dyes contains acidic groups, including sulfate groups and carboxylic groups. We can use this type of dyes to colour wool, silk and nylon through the establishment of an ionic bond between the acid group of the dye and the amine group of the fibre material.
Typically, acid dyes or anionic dyes are added to fibres at low pH values when it comes to the textile industry. Sometimes, these dyes can be used as food colourants as well. Some dyes are also important to stain organelles in the medical field.
What is the Difference Between Cationic and Anionic Dyes?
Dyes are substances we can use to colour other materials. There are dyes with different colours that can be used as desired. Cationic dyes are dye materials having components that make them dissociate into positively charged ions in an aqueous solution, while anionic dyes are dye materials having components that can make the dye molecule dissociate into negatively charged ions in an aqueous solution. The key difference between cationic and anionic dyes is that cationic dyes are basic, whereas anionic dyes are acidic.
The following infographic presents the differences between cationic and anionic dyes in tabular form.
Summary – Cationic vs Anionic Dyes
There are two major types of dyes as cationic dyes and anionic dyes. These two types are different from each other according to their chemical behaviours. The key difference between cationic and anionic dyes is that cationic dyes are basic, whereas anionic dyes are acidic.
Reference:
1. “Cationic Dyes.” Alfa Chemistry.
Image Courtesy:
1. “Chronic recurrent cholecystitis, HE 5” By Patho – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “2-naphthol red” By Esmu Igors – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwK2gqKaZmHqiusNmmKehn6O2pHnDspysZw%3D%3D