The key difference between carbonyl and ketone is that all carbonyl groups have a carbon atom with double bonded oxygen atom whereas the ketones have a carbonyl group attached to two alkyl groups.
Carbonyl group is a common functional group in organic chemistry with a wide range of reactivity. The two types of carbonyls we are familiar with are ketone and aldehydes.
CONTENTS
1. Overview and Key Difference
2. What is Carbonyl
3. What is Ketone
4. Side by Side Comparison – Carbonyl vs Ketone in Tabular Form
5. Summary
What is Carbonyl?
Carbonyl group is a functional group with double bonded oxygen to a carbon. Aldehydes and ketones are organic molecules with this group. The carbonyl group in an aldehyde always gets number one in the nomenclature, because it occurs at the end of the carbon chain. However, the carbonyl group of a ketone always locates in the middle.
Nature
According to the type of the carbonyl compound, nomenclature differs. “al” is the suffix that we use to name aldehydes whereas “one” is the suffix for ketones. Moreover, the carbon atom next to the carbonyl carbon is the α carbon, which has important reactivity due to the adjacent carbonyl.
Furthermore, the carbon atom in the carbonyl group is sp2 hybridized. Therefore, aldehydes and ketones have a trigonal planar arrangement around the carbonyl carbon atom. It is a polar group (electronegativity of oxygen is larger than carbon; therefore, carbonyl group has a large dipole moment); thus, aldehydes and ketones have higher boiling points compared to the hydrocarbons having the same weight.
However, these cannot make stronger hydrogen bonds like alcohols that result in lower boiling points than the corresponding alcohols. Moreover, because of the hydrogen bond formation ability, low molecular weight aldehydes and ketones are soluble in water. However, when the molecular weight increases, they become hydrophobic. Apart from that, the carbonyl carbon atom has a partial positive charge; hence, it can act as an electrophile. Therefore, these molecules easily undergo nucleophilic substitution reactions.
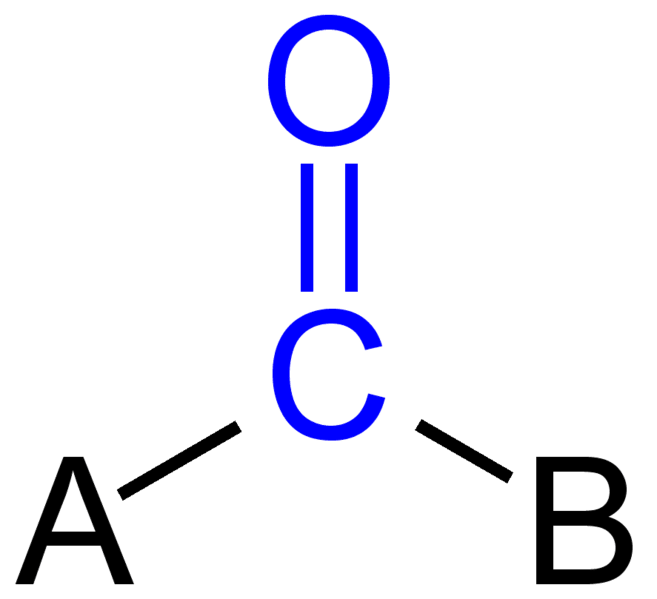
Figure 01: Structure of Carbonyl Group
The hydrogens attached to the carbon; next to the carbonyl group has an acidic nature, which accounts for various reactions of aldehydes and ketones. Compounds containing carbonyl groups are widely occurring in nature. Cinnamaldehyde (in cinnamon bark), vanillin (in the vanilla bean), camphor (camphor tree), and cortisone (adrenal hormone) are some of the natural compounds with a carbonyl group.
What is Ketone?
In ketones, the carbonyl group is situated between two carbon atoms. The general formula of a ketone is as follows;
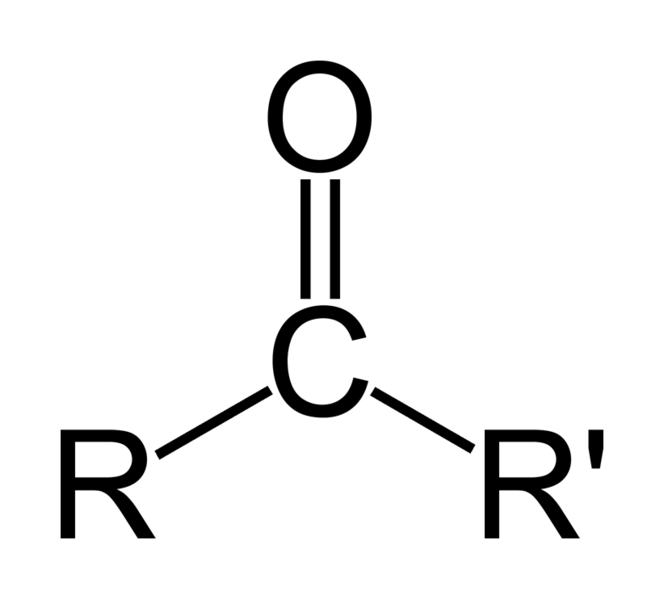
Figure 02: Structure of Ketone
“One” is the suffix used in ketone nomenclature. Instead of –e of the corresponding alkane we use “one”. Moreover, we can number the aliphatic chain in a way that gives the carbonyl carbon the lowest possible number. For example, we name the compound CH3COCH2CH2CH3 as 2-pentanone.
Furthermore, we can synthesize ketones from the oxidation of secondary alcohols, by ozonolysis of alkenes, etc. Ketones have the ability to undergo keto-enol tautomerism. And, this process happens when a strong base takes up the α-hydrogen (hydrogen attached to the carbon, which is next to the carbonyl group). The ability to release the α-hydrogen makes ketones more acidic than corresponding alkanes.
What is the Difference Between Carbonyl and Ketone?
Carbonyl group is a functional group in organic compounds in which a carbon atom has a double bonded oxygen atom, but a ketone is an organic compound in which the carbonyl group is attached to two alkyl groups. Therefore, the key difference between carbonyl and ketone is that all carbonyl groups have a carbon atom with double bonded oxygen atom whereas the ketones have a carbonyl group attached to two alkyl groups. We can denote a carbonyl group as-(C=O)- while a ketone as R’-C(=O)-R”.
Moreover, the carbonyl group in a ketone is always situated in the middle of a chain whereas the carbonyl group in an aldehyde can be located in the ends of a molecule. Therefore, another significant difference between carbonyl and ketone is that the carbonyl group can occur either in the middle of the molecule or at the end of the molecule while the carbonyl group of a ketone always occur at the middle of the molecule.
The below infographic on the difference between carbonyl and ketone provides more information about these differences.
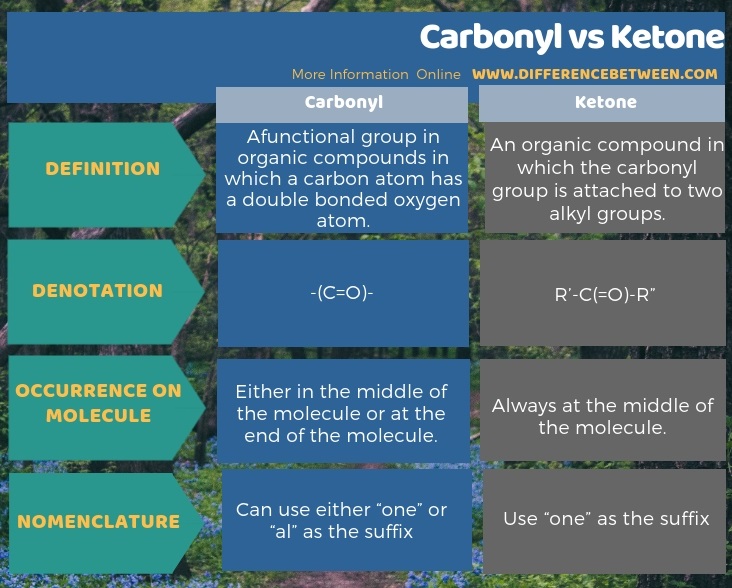
Summary – Carbonyl vs Ketone
Ketones are an example of organic molecules with a carbonyl functional group. The key difference between carbonyl and ketone is that all carbonyl groups have a carbon atom with double bonded oxygen atom whereas the ketones have a carbonyl group attached to two alkyl groups.
Reference:
1. Libretexts. “The Carbonyl Group.” Chemistry LibreTexts, National Science Foundation, 26 Nov. 2018. Available here
2. Brown, William H. “Ketone.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 27 June 2018. Available here
Image Courtesy:
1.”Carbonyl Group V.2″By Jü – Own work, (Public Domain) via Commons Wikimedia
2.”Ketone-general”By No machine-readable author provided. (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwKuZqKapoXqiusNmraxlm5rBsLrEaA%3D%3D