Calcium vs Calcium Citrate
The difference between Calcium and Calcium Citrate stems from the fact that Calcium Citrate is a product of Calcium, which is a reactive element. Calcium is an essential element for the proper function of the human body whereby the deficiency of the element may lead to various health complications. Calcium being a reactive element can form a variety of compounds and one such compound is Calcium Citrate. Calcium Citrate is the product of Calcium reacting with citric acid.
What is Calcium?
Calcium is a reactive element classified under alkaline earth metals belonging to the ‘s’ block of elements of the periodic table and 20th in atomic number. It is also abundant in the earth’s crust and as a dissolved ion in seawater. It also plays a major role in the proper function of the human body, being a main constituent of bones and teeth.
However, due to the reactivity of calcium, it is hard to find the element in isolation as it tends to form compounds with other anionic species. When calcium is needed in pure metal form, a calcium salt is often subjected to electrolysis. Calcium can also occur in a series of isotopes 40Ca, 42Ca, 43Ca, 44Ca, and 46Ca, which are found to be considerably stable. The history of Calcium dates back to several thousands of years BC with the discovery of limestone as a construction material. Calcium can be naturally found in sedimentary rocks in the forms of calcite, dolomite and gypsum. It also contributes to maintaining important climate cycles such as the carbon cycle. Calcium carbonate, calcium citrate, calcium nitrate, calcium sulfide, calcium chloride and calcium phosphate are some of the important compounds of calcium.
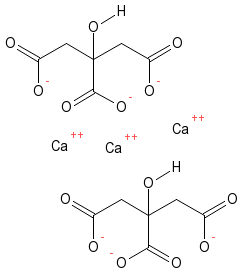
What is Calcium Citrate?
This is the salt that is produced by the reaction of Calcium with Citric Acid. Calcium Citrate is a lot similar to Sodium Citrate and is commonly used in the food industry as a food additive and preservative, and also to enhance flavor. It is a white powder by nature and has poor solubility in water. As mentioned above, Calcium plays a major role in the function of the human body; especially, in bone formation and maintenance. Therefore, when there is a Calcium deficiency (when the correct amount of Calcium is not taken in diet) Calcium is taken externally as supplements in the form of Calcium Citrate. It does not require stomach acids or food for its absorption. Hence, it is favored over other forms of calcium and is considered gentle on the stomach. If Calcium Carbonate was used, due to its basic properties, it would neutralize stomach acids but because Calcium Citrate is acidic it will not have any effect on stomach acids.
It is important to seek medical advice before taking any calcium supplement because, if in excess, it could lead to stones in kidneys or parathyroid gland disorders.
What is the difference between Calcium and Calcium Citrate?
• Calcium is an element whereas Calcium Citrate is a compound resulting from the reaction between Calcium and Citric Acid.
• Calcium element is very reactive, but Calcium Citrate is more stable as it is a compound.
• Calcium being a metal is basic in chemical nature whereas Calcium Citrate is an acid derivative.
• Calcium Citrate is used as a common dietary supplement of Calcium, but Calcium is not taken in pure elemental form.
Images Courtesy:
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwKWaoq2dYq6vsIyvqmabkaGwqsHMZpqirKKWwaZ7