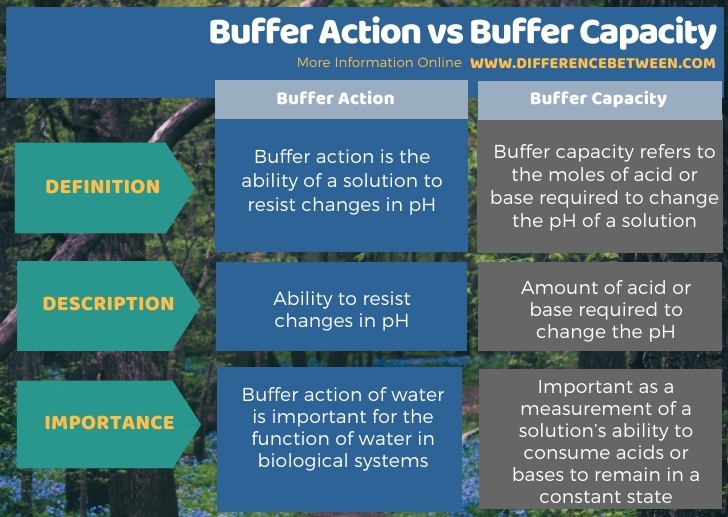
The key difference between buffer action and buffer capacity is that buffer action refers to the ability of a solution to resist changes in pH whereas buffer capacity refers to the moles of acid or base needed to change the pH of a solution.
A buffer solution is an aqueous solution made up of a weak acid and its conjugate base. The terms buffer action and buffer capacity describe properties of solutions that can act as buffers.
CONTENTS
1. Overview and Key Difference
2. What is Buffer Action
3. What is Buffer Capacity
4. Side by Side Comparison – Buffer Action vs Buffer Capacity in Tabular Form
5. Summary
What is Buffer Action?
Buffer action is the ability of a solution to resist changes in pH. The addition of a certain amount of an acid or a base to a buffer solution can change the pH of a buffer solution. Buffer action refers to the ability to remain unchanged upon addition of a small amount of acid or base. The solutions that can show this ability are known as buffer solutions or simply as buffers.
Furthermore, this phenomenon is very important; if we take water as an example, its ability to remain unchanged upon addition of acid or base to some extent helps to keep its value in the function of biological systems.
What is Buffer Capacity?
Buffer capacity refers to the moles of acid or base required to change the pH of a solution. It is a quantitative measurement regarding the resistance towards the pH changes on addition or reduction of hydroxide ions or hydrogen ions. We can calculate this value by dividing the amount of acid or base required to change the pH of the buffer by pH change and the volume of the buffer solution.

Figure 01: A Sample Graph Showing the Buffer Capacity of a System
A solution obtains this ability due to the consumption of the acid or base added to the buffer solution by the buffering agent present in that solution. These buffer solutions have an equilibrium reaction between the acid and their conjugate base or vice versa. Therefore, the p-H will not drastically change upon the addition of further acid or base to some extent as long as the buffering agent is not completely reacted (stays in the equilibrium). Generally, we can calculate the buffering capacity using titrimetric methods.
What is the Difference Between Buffer Action and Buffer Capacity?
A buffer solution is an aqueous solution made up of a weak acid and its conjugate base. The terms buffer action and buffer capacity are terms applied to solutions that can act as buffers. The key difference between buffer action and buffer capacity is that buffer action refers to the ability of a solution to resist changes in pH whereas buffer capacity refers to the moles of acid or base required to change the pH of a solution.
Below infographic summarizes the difference between buffer action and buffer capacity.
Summary – Buffer Action vs Buffer Capacity
A buffer solution is an aqueous solution made up of a weak acid and its conjugate base. The terms buffer action and buffer capacity are applied mainly regarding the solutions that can act as buffers. The key difference between buffer action and buffer capacity is that buffer action refers to the ability of a solution to resist changes in pH whereas buffer capacity refers to the moles of acid or base needed to change the pH of a solution.
Reference:
1. Bosch, E., and M. Rosés. “CHROMATOGRAPHY | Buffers for Chromatography and Electrophoresis.” Encyclopedia of Separation Science, 2007, pp. 1–12., doi:10.1016/b978-012226770-3/10696-x.
2. Subirats, X., et al. “Buffers for Reversed-Phase Liquid Chromatography☆.”Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2015, doi:10.1016/b978-0-12-409547-2.11547-1.
3. “Buffer Solution.” Wikipedia, Wikimedia Foundation, 12 Jan. 2020, Available here.
Image Courtesy:
1. “3533039” (Pixabay License) via Pixabay
2. “Buffer capacity” By Petergans (talk). – I Petergans (talk) created this work entirely by myself (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26u1J%2BdnqpdlrC1tc6nZJqmlGKvtrLFnqlmm5GlrqS107Jm