The key difference between azimuthal and principal quantum number is that azimuthal quantum number describes the angular momentum of an electron in an atom whereas the principal quantum number describes the size of an electron orbital.
Quantum numbers are values that are important in describing the energy levels of an atom. There are four quantum numbers we can use to describe the position of an electron in an atom. They are the principal quantum number, azimuthal quantum number, magnetic quantum number and spin quantum number.
CONTENTS
1. Overview and Key Difference
2. What is Azimuthal Quantum Number
3. What is Principal Quantum Number
4. Side by Side Comparison – Azimuthal vs Principal Quantum Number in Tabular Form
5. Summary
What is Azimuthal Quantum Number?
The azimuthal quantum number is the quantum number that describes the angular momentum of an electron in an atom. Therefore, we can also call it the orbital angular momentum quantum number. The letter “l” denotes azimuthal quantum number. Furthermore, this quantum number determines the shapes of an orbital in which an electron exists. It is the second of the set of four quantum numbers. Thus, we can name it as the second quantum number as well (because the four quantum numbers describe the quantum state of an electron in an atom). The equation that can relate the azimuthal quantum number with the angular momentum is as follows:
L2Ψ=h2l(l+1) Ψ
Where L2 is the orbital angular momentum operator, Ψ is wavefunction of the electron and h is the reduced plank constant. Here, I is always a positive integer. According to quantum mechanics, each energy level has different subshells. These subshells differ from each other in their shape and orientation. The subshells of an energy level are named as –I, 0 and +l.
| Azimuthal number | Denotation | Number of orbitals | Maximum number of electrons |
| 0 | s | 1 | 2 |
| 1 | p | 3 (=-1, 0, +1) | 6 |
| 2 | d | 5 (=-2, -1, 0, +1, +2) | 10 |
| 3 | f | 7 (=-3, -2, -1, 0, +1, +2, +3) | 14 |
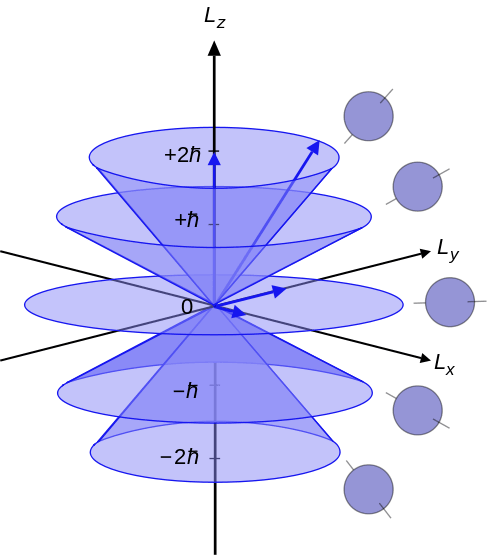
Figure 02: Azimuthal Quantum Numbers Ranging from –l, 0 to +l
What is Principal Quantum Number?
A principal quantum number is a quantum number that describes the main energy level in which an electron exists. We can denote it as “n”. Since it is the first of four different quantum numbers; we can call it the first quantum number as well. The value of the principal quantum number is a positive integer starting from 1, i.e. n=1, 2, 3,…
Higher the value of the principal quantum number, higher the energy of an electron; thus, the electron is loosely bound to the atom. That means; the high “n” values refer to higher energy levels. Moreover, for each “n” value, there are separate values for azimuthal quantum number, magnetic quantum number and spin quantum number. It is because each energy level has its own subshells, orbitals and electron pairs, respectively.
What is the Difference Between Azimuthal and Principal Quantum Number?
Quantum numbers are values that are important in describing the energy levels of an atom. There are four different quantum numbers, and the first two are the principal quantum number and azimuthal quantum number. The key difference between azimuthal and principal quantum number is that azimuthal quantum number describes the angular momentum of an electron in an atom, whereas the principal quantum number describes the size of an electron orbital. We can denote azimuthal quantum number as “l” and the principal quantum number as “n”.
Moreover, the azimuthal quantum number describes the angular momentum and the shape of an orbital, while the principal quantum number describes the energy level in which an electron exists.
Below infographic summarizes the difference between azimuthal and principal quantum number.
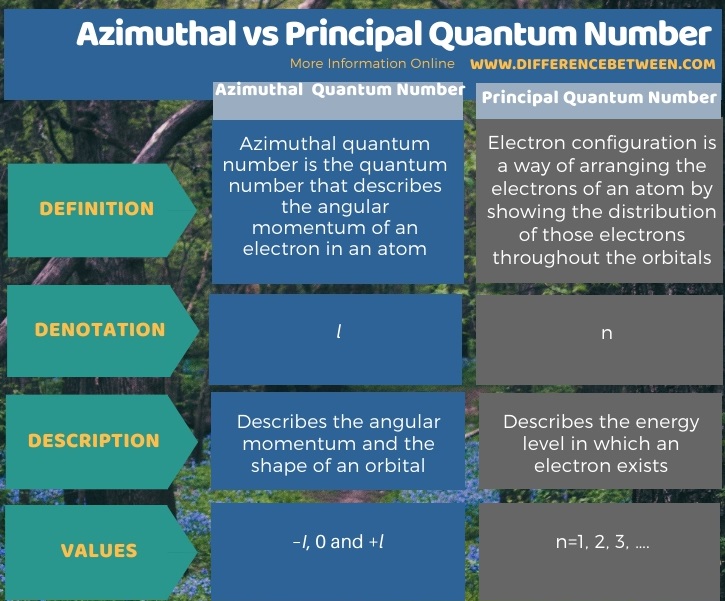
Summary – Azimuthal vs Principal Quantum Number
Quantum numbers are values that describe the energy levels of an atom. There are four different quantum numbers, and the first two are the principal quantum number and azimuthal quantum number. The key difference between azimuthal and principal quantum number is that azimuthal quantum number describes the angular momentum of an electron in an atom, whereas the principal quantum number describes the size of an electron orbital.
Reference:
1. Helmenstine, Anne Marie. “Azimuthal Quantum Number Definition.” ThoughtCo, Jun. 22, 2018, Available here.
2. Helmenstine, Anne Marie. “Principal Quantum Number Definition.” ThoughtCo, Nov. 6, 2019, Available here.
Image Courtesy:
1. “Vector model of orbital angular momentum” By Maschen – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26t2aKkrqyYlrlurc2dZKmqmaOwqrzApWSqrZGjwba5jKesppqVp3w%3D